
While the world's attention is often captured by sudden infectious outbreaks, a far more insidious and destructive threat is quietly dismantling Malaysia's future from within. This silent epidemic of non-communicable diseases (NCDs), including diabetes, hypertension, and obesity, has evolved from a

The daily task of moving a patient from a bed to a stretcher, a seemingly simple procedure, is a high-risk maneuver that has long plagued healthcare systems, contributing significantly to staff injuries and patient discomfort. At Mission Memorial Hospital, however, this routine is being
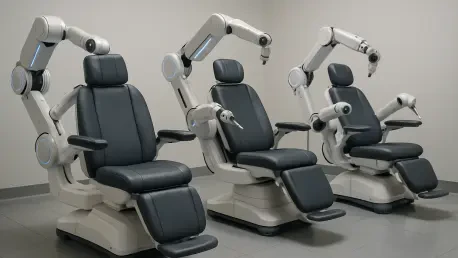
Making a significant statement at the imm cologne 2026 trade fair, the intelligent health technology brand VivaNova has officially unveiled its pioneering line of robotic health chairs, signaling a potential revolution in the ergonomic furniture industry. Backed by the considerable expertise of

A federal grand jury has unveiled a staggering indictment against two former top executives of a Colorado-based medical device company, alleging a complex fraud scheme that prosecutors claim bilked hundreds of millions of dollars from patients, insurance providers, and investors. The charges
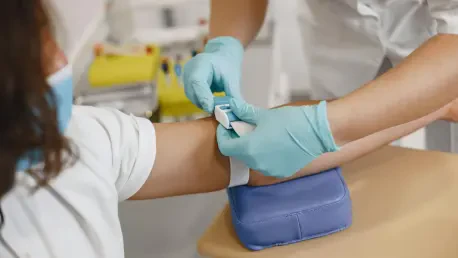
The routine act of having blood drawn, a cornerstone of modern diagnostics, often carries an undercurrent of anxiety for patients and presents a persistent operational challenge for healthcare systems grappling with staffing shortages and the need for procedural consistency. While medical

A groundbreaking strategic alliance announced on January 12, 2026, promises to untangle the notoriously complex web of healthcare billing through the power of advanced artificial intelligence. The partnership between frontier technology firm Rinova AI and revenue cycle management (RCM) leader Wise