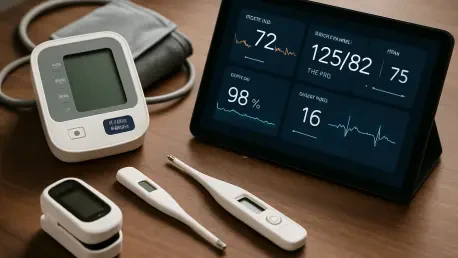
The delivery of healthcare is undergoing a fundamental transformation, shifting from the traditional confines of clinics and hospitals directly into patients' homes. This evolution is being powered by a wave of technological innovation, and at its forefront is the Remote Patient Monitoring (RPM)

Navigating the complexities of the healthcare system can be a daunting experience for anyone, but for autistic youth, this journey is often compounded by communication barriers and a historical lack of agency in their own medical decisions. A significant body of research is now challenging the

The promise of digital transformation in healthcare has long been overshadowed by the stark reality of physician burnout, a crisis largely fueled by the very Electronic Medical Records (EMRs) intended to streamline clinical practice. Instead of liberation, these systems have often brought digital

A profound transformation is reshaping modern medicine, shifting the central challenge from a scarcity of information to an overwhelming abundance of it, and nowhere is this paradigm shift more apparent than in the complex world of diabetes management. The proliferation of connected health devices

The global healthcare landscape is undergoing a seismic shift, with digital technologies fundamentally altering the very fabric of patient care and medical delivery. At the heart of this transformation is the telehealth market, a sector that is not merely growing but exploding, with projections

The prevailing landscape of personal health information is often a scattered and insecure puzzle, with critical data fragmented across numerous clinics, labs, and hospital systems, leaving individuals with little to no real authority over their own medical history. This disjointed approach not only