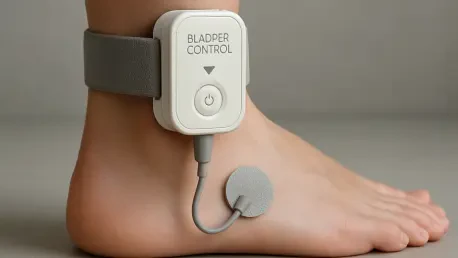
What if a tiny device, discreetly placed near the ankle, could end the daily struggle of unpredictable bladder urges for millions of people, offering a new lease on life? This isn’t a far-off dream but a reality in 2025, as Medtronic unveils a groundbreaking solution for urge urinary incontinence. The FDA approval of the Altaviva device marks a pivotal moment in pelvic health, promising a less invasive, long-lasting answer to overactive bladder (OAB)—a condition that silently disrupts countless lives. This innovation isn’t just a medical advancement; it’s a lifeline for those who’ve felt trapped by embarrassment and limitation.
Why Bladder Control Is a Silent Epidemic
Overactive bladder affects an estimated 30 million adults in the United States alone, cutting across age and gender to impact daily routines with sudden, uncontrollable urges. The condition often leads to social isolation, as sufferers avoid outings or activities due to fear of accidents. Beyond personal tolls, OAB places a significant burden on healthcare systems, with billions spent annually on management and treatment.
Many patients shy away from seeking help, deterred by stigma or the prospect of invasive procedures. Traditional therapies, ranging from medications with side effects to surgical implants, haven’t always met the need for accessibility and comfort. This gap in care highlights why a solution like Altaviva is not just timely but essential, addressing both physical and emotional barriers.
The Significance of FDA Approval for Altaviva
The green light from the FDA for Altaviva represents more than a regulatory win for Medtronic; it signals a shift toward patient-friendly innovation in medical technology. This approval, achieved after rigorous clinical trials demonstrating safety and efficacy, positions the device as a credible alternative in a market hungry for better options. It’s a testament to years of research aimed at transforming how OAB is managed.
Analysts predict that this milestone could redefine Medtronic’s standing in the pelvic health sector, potentially increasing market share in a space valued at over $1 billion annually. The approval also underscores a broader trend in healthcare—prioritizing treatments that minimize disruption to patients’ lives. For millions, this development isn’t just news; it’s a beacon of hope for regaining control.
How Altaviva Redefines Treatment with Cutting-Edge Design
At the heart of Altaviva’s appeal is its innovative approach to tibial neuromodulation, a method that targets the tibial nerve near the ankle with gentle electrical impulses. Unlike Medtronic’s earlier Interstim device, which requires implantation in the upper buttocks to stimulate the sacral nerve, Altaviva’s placement is far less daunting. This minimally invasive procedure, often completed in an outpatient setting, reduces both physical and psychological barriers to treatment.
The device’s standout feature is its remarkable 15-year battery life, a clear edge over competitors like Bluewind Medical’s Revi and Valencia Technologies’ offerings. This longevity means fewer follow-up surgeries for replacements, translating to lower costs and less hassle for patients. Combined with its discreet design, Altaviva emerges as a practical and durable solution for long-term bladder control.
Clinical studies have shown promising results, with over 70% of trial participants reporting significant improvement in urge control within months of implantation. For healthcare providers, this reliability offers a new tool to recommend with confidence. The design not only prioritizes efficacy but also aligns with modern demands for convenience in medical interventions.
Voices from the Field: Industry Leaders Weigh In
Medtronic CEO Geoff Martha has described the move to tibial neuromodulation as a “turning point” in making OAB therapy more approachable for a diverse patient base. During a recent earnings discussion, Martha emphasized that Altaviva’s ankle placement reduces the intimidation factor associated with traditional implants. His vision is clear: expand access while maintaining high standards of care.
Analysts at Leerink Partners share this optimism, noting that the device’s superior battery life and user-friendly approach could help Medtronic capture a significant portion of the underserved OAB market. Their forecasts suggest growth not just for the company but for the sector as a whole, as innovations like this encourage more patients to seek treatment. Such endorsements from industry experts add weight to the potential impact of this technology.
Beyond corporate and analytical perspectives, physicians on the front lines are expressing interest in integrating Altaviva into their practices. Dr. Susan Kline, a urologist based in Chicago, remarked that the device offers a “refreshing alternative” for patients hesitant about more invasive options. Her feedback reflects a growing sentiment among providers eager to adopt solutions that prioritize patient comfort.
Practical Impacts: What This Means for Patients and Doctors
For individuals battling OAB, Altaviva introduces a tangible opportunity to reclaim normalcy with a treatment that fits seamlessly into life. Patients are encouraged to consult their healthcare providers to determine if this device suits their needs, weighing factors like lifestyle and medical history against other therapies. The simplicity of the implantation process—often requiring just a short outpatient visit—makes it an attractive first step for many.
Healthcare professionals, meanwhile, gain a versatile tool to address a common yet complex condition. Training programs rolled out by Medtronic aim to equip doctors with the knowledge to perform the procedure and counsel patients effectively. This support ensures that the transition to offering Altaviva is smooth, fostering confidence in both providers and those they serve.
Access remains a key consideration as Medtronic scales up distribution in 2025 and beyond. Updates on insurance coverage and regional availability will be crucial for patients eager to explore this option. Additionally, the company’s ongoing restructuring of its pelvic health division signals a commitment to broadening reach, ensuring that more people can benefit from this advancement over time.
Reflecting on a Milestone in Pelvic Health
Looking back, Medtronic’s journey to secure FDA approval for Altaviva stood as a defining chapter in the fight against overactive bladder challenges. The device’s arrival marked a shift in how such conditions are approached, emphasizing minimal intrusion and maximum impact. It was a moment that reshaped expectations for millions.
For those affected, the next steps involve staying informed about availability and discussing candidacy with trusted medical professionals. Providers, too, have a role in advocating for wider access and integrating this technology into comprehensive care plans. As the landscape of pelvic health continues to evolve, the focus remains on ensuring that innovations like Altaviva reach every corner of need, paving the way for sustained improvements in quality of life.