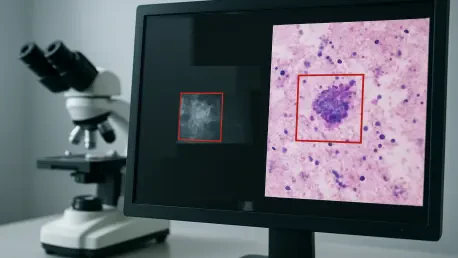
The convergence of artificial intelligence with medical imaging is fundamentally reshaping the landscape of oncological diagnostics, representing a quantum leap beyond incremental improvements in care. This technological fusion introduces an unprecedented level of precision and efficiency into the fight against cancer, functioning not as a replacement for human clinicians but as a powerful augmentation tool. By enhancing the analytical capabilities of radiologists, reducing the cognitive burden of reviewing vast quantities of images, and introducing a scale of data processing previously unimaginable, AI is poised to redefine the standards of early detection and intervention. The foundation for this revolution is built on the validated performance of new AI models that consistently surpass conventional diagnostic protocols, as demonstrated in a landmark study highlighted in Nature Communications Medicine. These systems can identify subtle, cancerous lesions often missed by experienced specialists, addressing the long-standing challenges of sensitivity and specificity in screening. By achieving superior accuracy, this technology promises to identify malignancies at their earliest, most treatable stages, arriving at a critical juncture as global healthcare systems confront the reality of rising cancer incidence rates.
The Technological Engine of a Diagnostic Revolution
At the heart of this diagnostic evolution are convolutional neural networks (CNNs), a sophisticated form of deep learning specifically engineered to process and interpret visual information. These algorithms undergo an intensive training phase, analyzing millions of annotated medical images, from mammograms to CT scans, to learn the complex patterns indicative of disease. Through this rigorous training, the networks learn to autonomously differentiate between benign and malignant tissue by recognizing minute variations in texture, density, and morphology that often escape the human eye. This capability marks a significant departure from earlier, simpler computer-aided detection (CAD) tools, which relied on predefined rules and basic pattern matching. In contrast, contemporary deep learning models develop their own complex, hierarchical understanding of image features, building from low-level details like pixel intensity to high-level conceptual features that correlate with specific pathologies. This layered, self-taught approach is the critical factor enabling modern AI to not only approach but, in documented cases, exceed human-level diagnostic performance, making it a true game-changer in the field of oncology.
The validation of these powerful systems is a meticulous and data-driven process, grounded in established clinical metrics to ensure their reliability and effectiveness in real-world settings. A pivotal study, for example, evaluated AI performance across thousands of cases, measuring key indicators such as sensitivity, specificity, and the area under the receiver operating characteristic curve. The results were striking, with AI models achieving sensitivity rates exceeding 95% for certain cancers while simultaneously posting false positive rates significantly lower than those associated with traditional screening methods. The clinical translation of these metrics is profound: higher sensitivity leads to fewer missed diagnoses and facilitates earlier, more effective treatment interventions. At the same time, lower false positives prevent the patient anxiety, stress, and healthcare costs associated with unnecessary follow-up procedures like biopsies. This dual improvement in accuracy and efficiency underscores the transformative potential of AI to enhance patient outcomes and optimize healthcare resources, solidifying its role as an indispensable tool in modern diagnostics.
Navigating the Path to Widespread Adoption
Despite their demonstrated technical success and immense potential, significant hurdles stand in the way of the widespread clinical adoption of these AI systems. A primary challenge lies in the regulatory domain, where governing bodies such as the U.S. Food and Drug Administration (FDA) are tasked with developing entirely new evaluation frameworks tailored to the unique nature of machine learning algorithms. Unlike static medical devices, which have a fixed design and function, these AI systems can learn and evolve over time. This dynamic characteristic necessitates the creation of protocols for ongoing monitoring, validation, and potential retraining to ensure their long-term safety, efficacy, and fairness. Furthermore, the integration of these sophisticated technologies into existing healthcare infrastructure presents a major logistical and financial challenge. Hospitals and clinics must make substantial investments in the required computational resources, including high-performance computing power and secure, large-scale data storage systems capable of handling the massive datasets required for AI operations, creating a significant barrier to entry for many institutions.
Beyond the regulatory and infrastructural challenges, the human element remains a critical component for successful implementation. Radiologists and other clinicians require comprehensive and ongoing training to effectively utilize these AI-assisted tools, enabling them to understand both their immense capabilities and their inherent limitations. This education is essential for fostering trust and ensuring that practitioners can properly interpret AI-generated insights within the broader clinical context of a patient’s case. The design of the human-machine interface is also paramount; the systems must present their complex analytical output in a clear, intuitive, and actionable format that supports, rather than complicates, clinical decision-making. If the interface is cumbersome or the data is presented opaquely, the risk of misinterpretation or underutilization increases, potentially negating the benefits of the technology. Ultimately, a seamless and synergistic collaboration between the human expert and the AI tool is the goal, requiring a thoughtful approach to workflow integration, user experience design, and professional development.
A Collaborative Future in Oncology
This technological evolution concurrently redefined the role of the radiologist and other clinicians, shifting their focus toward more consultative and strategic functions. As AI systems handled a greater share of routine diagnostic tasks, human experts were able to dedicate their expertise to interpreting the most complex and ambiguous cases, performing crucial quality assurance on AI outputs, collaborating more deeply on multidisciplinary treatment planning, and engaging in more meaningful patient communication. This synergy between human and artificial intelligence represented the optimal paradigm for the future of medicine. The AI excelled at tireless, high-volume pattern recognition across immense datasets, while the human practitioner provided the essential clinical context, ethical judgment, and holistic wisdom that came only from years of experience. This collaborative model exemplified how technology could augment human capabilities to advance medicine’s core mission: improving health outcomes and alleviating suffering by enabling earlier, more precise, and more personalized care for every patient.