

Navigating a New Era of Virtual Care The global telehealth landscape has shifted from a period of emergency-driven expansion to a phase of rigorous market consolidation where only the most adaptable platforms survive. Teladoc Health, once the undisputed leader of the remote care boom, now faces the

The daunting reality of managing specialized medical care at home often hinges on the availability of industrial-grade equipment that remains out of reach for the average household budget. In Bermuda, the transition from clinical hospital environments to the comfort of a private residence has

The landscape of behavioral healthcare has undergone a radical transformation as the traditional therapist’s couch is increasingly supplemented, or even replaced, by the sophisticated algorithms and intuitive interfaces of modern smartphone applications. This transition represents a fundamental

The implementation of mobile medical units across the Denver metropolitan area represents a sophisticated shift in how healthcare providers address the specific needs of vulnerable populations in 2026. By transitioning away from traditional, static clinical environments, organizations are now able

In a landmark transaction set to reshape the medical technology landscape, global life sciences conglomerate Danaher Corporation has officially announced its definitive agreement to acquire Masimo, a pioneer in advanced patient monitoring technologies. The all-cash deal, valued at a substantial
The promise of artificial intelligence in radiology is immense, but many healthcare organizations are discovering a frustrating gap between an AI model's advertised performance and its actual effectiveness in their own clinical environment. This "performance drift" happens because an algorithm

The modern clinical environment presents a significant paradox where physicians are surrounded by an unprecedented volume of data within the electronic health record, yet they often struggle to access the precise, evidence-based information needed at the moment of a critical decision. This deluge
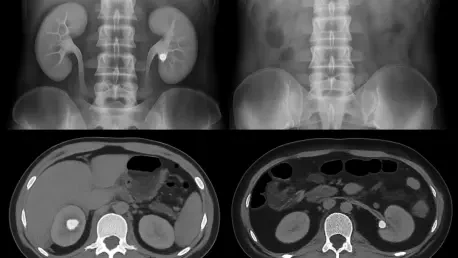
The intense, debilitating pain of a kidney stone often sends patients rushing to the emergency room, where the primary goal is a swift and accurate diagnosis to guide immediate treatment, but this urgent search for answers frequently involves a hidden, long-term risk. The go-to diagnostic tool, the
For millions of families, the journey to an Alzheimer's diagnosis has long been a frustrating and arduous path paved with uncertainty, invasive procedures, and prolonged waiting periods for definitive answers. The traditional diagnostic toolkit, heavily reliant on expensive brain scans and

The landscape of clinical research is currently navigating a complex and often fragmented technological terrain, where sponsors and contract research organizations (CROs) frequently juggle a patchwork of disparate systems to manage the lifecycle of a trial. This siloed approach creates significant
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53