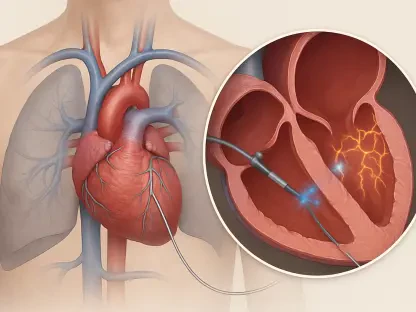
In a significant advancement for neonatal medicine, Abbott has secured dual regulatory clearance for its Amplatzer Piccolo Delivery System from the U.S. Food and Drug Administration and a CE mark in the European Union. This approval marks a pivotal moment in treating patent ductus arteriosus (PDA), a life-threatening heart defect common in premature infants. The new system is specifically engineered to deploy the company’s existing pea-sized Amplatzer Piccolo Occluder, representing a major leap forward in procedural safety and precision for the most fragile patients.
From Groundbreaking Occluder to Enhanced Delivery: The Evolution of PDA Treatment
To appreciate this new clearance, understanding the challenge it addresses is key. Patent ductus arteriosus is a persistent opening between two major blood vessels near the heart that often fails to close in premature infants, leading to severe complications. Affecting up to 30% of infants born under 2.5 kilograms, PDA historically required either unreliable medication or high-risk open-heart surgery. While Abbott’s 2019 Amplatzer Piccolo Occluder was a game-changing, minimally invasive solution, real-world application paved the way for this newly refined delivery system.
Inside the Innovation: A Closer Look at the Amplatzer Piccolo System
Driven by Physician Feedback: Prioritizing Safety and Precision
The new Amplatzer Piccolo Delivery System is the direct result of invaluable feedback from pediatric cardiologists. While the original occluder had a high 95.5% implant success rate, practitioners identified a need to mitigate risks like device migration. Abbott’s new system directly addresses this concern with a redesigned mechanism that is more intuitive and stable, shifting the focus from a successful implant to a perfected, safer procedural experience.
Enhancing a Minimally Invasive Breakthrough
The core benefit of the Amplatzer Piccolo Occluder is its minimally invasive nature. The new delivery system amplifies this advantage by giving physicians enhanced control and stability during the transcatheter procedure. This refinement is designed to make occluder deployment more predictable and secure, which can translate into shorter procedure times, reduced anesthesia exposure, and a lower likelihood of complications for vulnerable infants.
Strengthening a Key Growth Engine: The Business Impact for Abbott
Beyond its clinical importance, this dual clearance is a significant strategic victory for Abbott. It strengthens the company’s dominant structural heart portfolio, a primary growth driver for its medical device unit, which saw 13.6% expansion in the third quarter. By refining its flagship products based on clinical feedback, Abbott reinforces its market leadership and builds deeper trust within the medical community, ensuring its technologies remain the standard of care.
The Future of Pediatric MedTech: A Trend Toward Iterative Innovation
The clearance of this delivery system signals a broader trend in the medical device industry: a shift toward continuous, iterative innovation. Rather than one-off inventions, companies increasingly recognize that post-market surveillance and collaboration with clinicians are crucial for enhancement. This feedback loop is becoming the engine for next-generation devices optimized not just for efficacy but for superior safety, usability, and patient outcomes.
Key Takeaways for Clinicians and the Medical Device Industry
This achievement offers clear takeaways. For clinicians, it highlights the value of adopting technologies that minimize procedural risk and improve workflow, making a compelling case for the new system’s integration into standard practice. For the medical device industry, it exemplifies the power of a customer-centric design philosophy. Actively incorporating physician feedback is a critical strategy for developing market-leading products that meet real-world clinical needs.
A Small Device with a Monumental Impact on Neonatal Health
In conclusion, the clearance for Abbott’s Amplatzer Piccolo Delivery System is far more than a routine update. It represents a significant milestone in providing safer treatments for the world’s most vulnerable patients. By refining an already successful technology based on direct clinical insights, Abbott has elevated the standard of care for premature infants with this heart condition, reinforcing the profound impact that thoughtful, collaborative innovation can have on medicine.