I’m thrilled to sit down with James Maitland, a renowned expert in robotics and IoT applications in medicine, whose passion for integrating cutting-edge technology into healthcare has made significant waves in the industry. Today, we’re diving into a critical topic: the recent recall of carotid artery stents by a major medical device manufacturer due to a manufacturing defect. Our conversation will explore the reasons behind the recall, the impact on patient safety, the scale of the issue, and the steps being taken to address it. James brings a unique perspective on how technology and regulation intersect in such scenarios, making this a discussion you won’t want to miss.
Can you walk us through what prompted the recall of these carotid artery stents and the specific manufacturing issue at play?
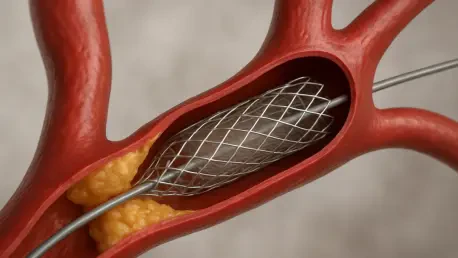
Certainly, Jan. The recall of the Carotid Wallstent Monorail Endoprosthesis devices stems from a manufacturing defect that resulted in an inner lumen of the stent delivery system being smaller than specified. This flaw creates unexpected resistance when physicians attempt to withdraw the delivery system after placing the stent. It’s a significant concern because this resistance can complicate the procedure and potentially lead to serious complications for the patient.
How does this defect in the delivery system impact the procedure itself for physicians and patients?
When the delivery system resists withdrawal, it can disrupt the entire procedure. Physicians expect a smooth process when navigating the stent over a guidewire or embolic protection device. This defect can cause delays or require additional interventions to resolve the issue, which increases the risk of complications like damaging the stent or even the blood vessel itself. For patients, this means a higher chance of adverse events during what should be a routine intervention.
With over 26,000 devices recalled globally, including more than 1,300 in the U.S., how is the scale of this issue being managed?
The scale is indeed substantial, with 26,570 devices affected worldwide and 1,333 in the United States. Managing this requires a robust strategy to track down every single unit. The manufacturer has initiated direct communication with healthcare facilities to identify where these devices were distributed. The focus is on ensuring that none of these stents remain in use by urging facilities to pull them from inventory immediately and coordinating their return.
There were reports of six cases requiring additional intervention due to this defect. Can you shed light on what these interventions entailed?
Yes, in those six cases reported as of late July, physicians encountered significant resistance during the withdrawal of the delivery system, necessitating further steps to resolve the issue. These interventions likely involved additional tools or techniques to safely remove or adjust the system without causing harm. It’s a stressful situation for both the medical team and the patient, as it extends the procedure time and introduces uncertainty.
What are the most serious risks this defect poses to patients undergoing these stent placements?
The risks are quite severe. A defective delivery system can lead to injury of the blood vessel, damage to the stent itself, or even the release of debris that could travel to the brain and trigger a stroke. Stroke is highlighted as the most critical potential outcome, and while no deaths have been linked to this issue so far, the possibility of such serious harm underscores the urgency of the recall.
How are healthcare providers being informed and supported to ensure these defective devices aren’t used?
The manufacturer acted quickly by instructing customers last month to stop using the affected devices. This involved sending out detailed communications to healthcare providers, likely through urgent recall notices, outlining the defect and the associated risks. Beyond just informing, there’s an emphasis on confirming that these stents are removed from hospital shelves, often through follow-up checks or inventory audits to ensure compliance.
Could you describe the process for healthcare facilities to return these recalled stents and any challenges they might face?
The return process typically involves facilities isolating the affected devices, packaging them according to specific guidelines provided by the manufacturer, and shipping them back through a designated channel. Challenges can include logistical issues, especially for smaller facilities with limited staff or resources to handle such recalls promptly. There’s also the task of ensuring every unit is accounted for, which can be time-consuming but is critical to patient safety.
Given that no deaths were linked to this issue as of late July, are there any updates or ongoing efforts to monitor patient outcomes?
As of the latest information I have, there were no reported deaths connected to this defect by the end of July. However, monitoring continues to be a priority. The manufacturer and regulatory bodies like the FDA are likely keeping a close watch on patient outcomes through post-market surveillance and encouraging healthcare providers to report any adverse events. This ongoing vigilance is crucial to catch any delayed complications that might emerge.
Looking ahead, what is your forecast for how such manufacturing defects in medical devices might be prevented or better managed in the future?
I believe we’re moving toward a future where advanced technologies like IoT and real-time data analytics will play a bigger role in quality control during manufacturing. Imagine sensors embedded in production lines that detect deviations in specifications instantly, preventing defective units from ever reaching the market. Additionally, stronger collaboration between manufacturers, regulators, and healthcare providers can streamline recall processes and enhance transparency. We also need to invest in training and simulation for physicians to handle unexpected issues during procedures. The goal is to build a system where patient safety is safeguarded at every step, from production to post-market monitoring.