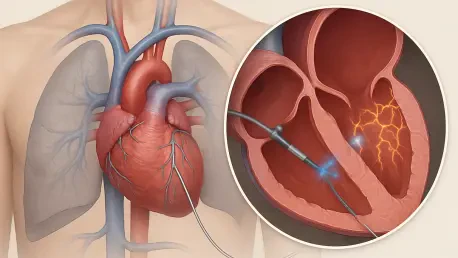
The recent Atrial Fibrillation Symposium in Boston served as a critical battleground for innovation, where groundbreaking clinical data reshaped the landscape of treatment for a condition affecting millions worldwide. At the heart of this evolution is the rapid ascent of pulsed field ablation (PFA), a non-thermal energy source poised to replace traditional methods by offering a potentially safer and more effective way to electrically isolate the pulmonary veins where erratic signals often originate. While established medical technology firms showcased significant progress, a lesser-known player unveiled results so compelling they suggest a fundamental leap forward in how this complex arrhythmia may be managed. The symposium’s key takeaway was not just the validation of PFA as a whole, but the emergence of a specific nanosecond-based approach that is now forcing the entire electrophysiology community to reconsider the very definition of a successful long-term outcome for patients living with atrial fibrillation.
Abbott Cements Its Role with a Versatile Two-Pronged Strategy
Abbott demonstrated its formidable presence in the electrophysiology space by presenting robust one-year outcomes from the pivotal IDE study of its Volt PFA System. The data, simultaneously published in a leading clinical journal, revealed an impressive 84.2% rate of freedom from arrhythmia recurrence in patients with paroxysmal atrial fibrillation, a figure the company characterized as industry-leading. For the more challenging cohort of patients with persistent AFib, the system maintained a strong 68% success rate at the one-year mark. These results are particularly significant given Volt’s recent FDA approval and the initiation of commercial procedures in the United States, placing Abbott on solid footing to compete against existing PFA systems from major rivals. The findings from the VOLT-AF study provide a strong foundation for the system’s adoption, validating its efficacy and durability in a market that is increasingly shifting its allegiance from thermal to pulsed-field energy sources for cardiac ablation.
Beyond its dedicated PFA platform, Abbott also underscored its strategic depth by showcasing strong six-month results for its TactiFlex Duo catheter. This device offers a unique hybrid approach, empowering physicians to deliver both PFA and traditional radiofrequency (RF) energy, which provides critical flexibility for treating more complex arrhythmia cases. The CE mark trial for this catheter reported an 81% freedom from recurrence among paroxysmal AFib patients, reinforcing its clinical utility. Further highlighting its potential, Abbott has completed enrollment in a U.S. IDE trial and received an FDA breakthrough device designation for the catheter’s use in treating ventricular tachycardia, a different and often more dangerous heart rhythm disorder. Christopher Piorkowski, Abbott’s Chief Medical Officer for electrophysiology, framed these collective advancements as a clear affirmation of the company’s mission to elevate the standard of care, offering a comprehensive toolkit that allows for tailored treatment strategies based on individual patient anatomy and arrhythmia characteristics.
Nanosecond Precision Signals a Potential Paradigm Shift
While Abbott’s advancements were significant, the most disruptive news from the symposium may have come from Pulse Biosciences. The company presented one-year, first-in-human trial data for its nPulse cardiac catheter that captivated attendees and analysts alike. The trial, which included 150 patients, reported a remarkable 96% procedural success rate at the one-year follow-up. This level of durable success prompted Mizuho analyst Anthony Petrone to remark that the data “easily exceeded” not only the company’s own preliminary three-month results but also the one-year durability data from competing PFA systems currently on the market. David Kenigsberg, Pulse’s Chief Medical Officer of electrophysiology, noted that these outcomes surpassed even their internal expectations, standing in stark contrast to the 20% to 25% recurrence rate commonly anticipated in the field, suggesting a potential breakthrough in long-term treatment efficacy for atrial fibrillation.
The exceptional performance of the nPulse system appears to stem from its core technological differentiator: the use of nanosecond-duration energy pulses. This is a fundamental departure from the microsecond pulses employed by Abbott and other competitors. This ultra-rapid energy delivery may be the key to achieving more durable and effective cardiac tissue ablation, leading to the superior outcomes observed in the trial. In addition to its high efficacy, the technology also demonstrated a strong safety profile and procedural efficiency, with rapid treatment times and minimal adverse effects. The primary safety endpoint revealed only two patients in the study experienced serious adverse events. Buoyed by this compelling feasibility data, Pulse Biosciences has announced its plans to advance to a pivotal IDE study and expand patient access in both the United States and Europe, positioning its novel nanosecond PFA technology as a powerful new force that could significantly raise the bar for what is considered a successful AFib treatment.
A Look Ahead at the Evolving Treatment Landscape
The data presented at the symposium solidified PFA’s role as a dominant force in modern electrophysiology while simultaneously introducing a new layer of technological competition. Abbott’s dual-modality strategy confirmed the value of providing physicians with a versatile and comprehensive toolkit capable of addressing both straightforward and complex cases with proven platforms. The strong, durable results from its Volt PFA system and the flexibility of the TactiFlex Duo catheter ensured its continued leadership in a highly competitive market. These innovations provided reassurance that incremental, well-validated advancements continue to improve patient outcomes and procedural safety. The market’s positive reception to these established players highlighted the importance of robust clinical evidence and a track record of reliability in driving the adoption of new medical technologies for treating a condition as prevalent as atrial fibrillation.
In contrast, the emergence of Pulse Biosciences’ nanosecond technology served as a powerful reminder that disruptive innovation can redefine an entire field. The unprecedented 96% one-year success rate shifted the conversation from incremental improvements to the possibility of a transformational leap in long-term efficacy. This development suggested that the subtle, yet critical, differences in energy delivery mechanisms—from microsecond to nanosecond pulses—could unlock a new level of performance and durability in cardiac ablation. As Pulse Biosciences moves toward pivotal trials, the industry has been left to contemplate a future where a novel approach may not just compete with, but potentially supersede, the current generation of PFA systems. This has set the stage for a new chapter in AFib treatment, where the quest for the ideal energy source has become more nuanced and more exciting than ever before.