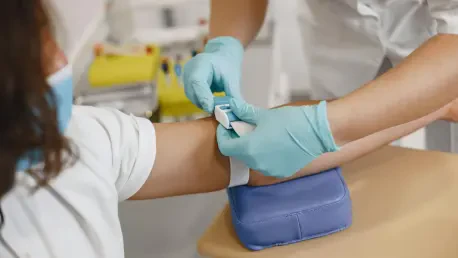
The routine act of having blood drawn, a cornerstone of modern diagnostics, often carries an undercurrent of anxiety for patients and presents a persistent operational challenge for healthcare systems grappling with staffing shortages and the need for procedural consistency. While medical technology has seen monumental leaps in imaging, surgery, and treatment, the fundamental process of phlebotomy has remained largely unchanged for decades, heavily reliant on the manual skill and experience of human technicians. This reliance introduces variability, with success rates fluctuating based on patient physiology and practitioner expertise. Now, a new wave of innovation is poised to transform this universal procedure, leveraging the precision of robotics and the intelligence of AI to create a more standardized, efficient, and patient-friendly experience. A fully autonomous device developed by the Dutch medical robotics company Vitestro, known as Aletta, is at the forefront of this shift, aiming to solve long-standing issues in preanalytical workflows and establish a new benchmark for diagnostic accuracy.
A New Standard in Automated Diagnostics
The Technological Core of Aletta
The Aletta device represents a significant leap in clinical automation, integrating a sophisticated suite of technologies to perform the entire phlebotomy process without direct human guidance. Its core functionality is built upon a fusion of advanced imaging and precision robotics, governed by an intelligent AI system. To begin the procedure, the device uses near-infrared scanning to create a detailed map of the patient’s veins, identifying potential targets that are invisible to the naked eye. This initial mapping is then enhanced by ultrasound technology, which provides a three-dimensional view of the selected vein, measuring its depth and diameter with submillimeter accuracy. To ensure optimal blood flow before insertion, the system employs Doppler ultrasound to confirm that the target vessel is viable. Only after this multimodal confirmation does the robotic arm, equipped with a standard needle, execute the venipuncture. The entire mechanical process, from applying a tourniquet to collecting the required samples and finally placing a bandage, is performed with a level of precision designed to minimize error and maximize success, fundamentally reimagining a procedure that has long depended on human touch and sight.
The clinical efficacy of this automated system is demonstrated through impressive performance metrics that exceed many industry standards for manual procedures. Aletta has achieved a 95% first-stick success rate in clinical trials, a figure that stands out when compared to the 80-89% success rates often documented for manual blood draws, particularly in patients with difficult-to-access veins. This high rate of success directly translates to a better patient experience by reducing the need for multiple attempts. Furthermore, the device has recorded an exceptionally low hemolysis rate of just 0.6%. Hemolysis, the rupture of red blood cells, can compromise sample quality and lead to inaccurate test results, often requiring a redraw. By staying well below the widely accepted industry benchmark of 2%, Aletta ensures the integrity of the collected samples, contributing to more reliable diagnostic outcomes and reducing downstream costs associated with retesting. The procedure is also remarkably efficient, completed in under two minutes, with the needle strategically concealed from the patient’s view to help alleviate procedural anxiety.
Redefining the Patient and Staff Experience
The development of Aletta is a direct response to some of the most pressing challenges facing modern healthcare systems, most notably the chronic global shortage of skilled phlebotomists. As the demand for diagnostic testing continues to rise, laboratories and hospitals struggle to maintain adequate staffing levels, leading to longer patient wait times and increased pressure on existing personnel. This robotic solution introduces a new paradigm of efficiency, enabling a single supervisor to oversee the operation of up to three units simultaneously. This force-multiplying effect allows high-volume environments, such as outpatient clinics and central laboratories, to significantly increase their throughput without a proportional increase in staff. By automating this routine but critical task, the system frees highly trained medical professionals to dedicate their time and expertise to more complex patient care activities, optimizing the allocation of human resources. The result is a more streamlined workflow that benefits both the institution and the patients it serves.
Beyond its operational benefits, the Aletta system was engineered with a strong emphasis on patient-centric design, aiming to make the blood draw experience as comfortable and stress-free as possible. This focus is validated by data from clinical trials, which show high levels of patient acceptance and satisfaction. An overwhelming 98% of participants in these studies indicated their willingness to undergo the robotic procedure again, signaling strong trust in the technology. Importantly, 85% of these patients reported that the pain experienced was comparable to or even less than that of a traditional manual blood draw. The system’s safety profile is equally robust, with minimal adverse events recorded during trials, underscoring its reliability. By combining procedural precision with thoughtful design elements, such as hiding the needle from sight, the device directly addresses common sources of patient fear and discomfort, setting a new standard for a more positive and less intimidating diagnostic experience.
Navigating the Path to Global Adoption
Securing Regulatory and Commercial Milestones
Vitestro has made significant strides in bringing its innovative technology from the laboratory to clinical practice, successfully navigating complex regulatory landscapes. The Aletta device achieved a pivotal milestone in 2024 by securing CE marking, a certification that confirms its compliance with European Union health, safety, and environmental protection standards. This approval authorized its commercial launch across the EU, and the system is already in clinical use at several hospitals in the Netherlands, where it is demonstrating its value in real-world settings. Looking toward the North American market, the company is actively pursuing approval from the U.S. Food and Drug Administration (FDA). This process involves a series of multi-center clinical studies conducted in partnership with prominent U.S. health systems. These trials, scheduled to begin in 2027, are designed to generate the robust data required for a formal pre-market application, which is anticipated to be submitted soon after their conclusion.
Building trust and transparency is a central component of Vitestro’s strategy for introducing Aletta to the global healthcare market. The company has actively engaged in public demonstrations and pilot programs to familiarize both clinicians and patients with the technology, helping to demystify the concept of an autonomous blood draw. By allowing stakeholders to see the device in action and understand the rigorous safety and precision measures built into its design, Vitestro is proactively addressing potential concerns about automation in patient-facing roles. This educational approach positions Aletta not merely as a product but as a new, responsible standard in the preanalytical phase of diagnostics. The goal is to foster a broad-based acceptance that paves the way for widespread adoption, ensuring that the technology is viewed as a reliable partner in delivering high-quality healthcare rather than just a replacement for human labor. This focus on stakeholder engagement is crucial for integrating such a disruptive innovation into established clinical workflows.
A New Chapter in Medical Diagnostics
The journey of this autonomous phlebotomy system from concept to clinical implementation marked a significant step forward in healthcare automation. Its successful deployment in European hospitals provided tangible evidence of how robotics and AI could address systemic challenges like staffing shortages and procedural variability. The high rates of patient acceptance and clinical efficacy observed in initial studies helped build a strong case for its broader adoption, demonstrating that technology could enhance, rather than depersonalize, the patient experience. The careful navigation of regulatory pathways in both Europe and the United States laid the groundwork for a future where such automated systems could become a standard feature in hospitals and laboratories worldwide. This progress suggested a shift in how routine medical tasks were perceived and managed, pointing toward a future where human expertise was augmented by machine precision.