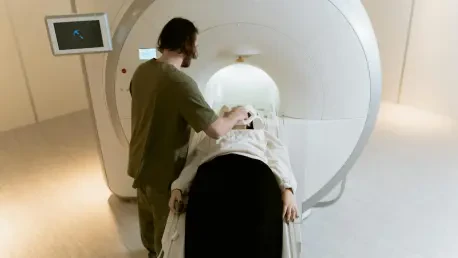
In a shocking turn of events on August 27, a serious safety incident at AZ Sint-Blasius Hospital in Dendermonde, East Flanders, Belgium, left eight patients ill after undergoing computed tomography (CT) scans, raising urgent concerns about medical safety protocols and the integrity of critical substances used in such procedures. The root cause was traced to contamination of a contrast agent, Iomeron 400, with Klebsiella bacteria, resulting in severe health complications for those affected. This alarming situation has sparked intense scrutiny over how such a breach could occur in a controlled hospital environment, prompting investigations by both the hospital and the manufacturer. Beyond the immediate impact on the patients, this incident underscores broader vulnerabilities in radiology practices and the handling of critical medical substances, setting the stage for a deeper examination of infection control measures.
Hospital Response and Initial Findings
Immediate Aftermath and Accountability
The rapid response from AZ Sint-Blasius Hospital following the incident on August 27 demonstrated a commitment to addressing the crisis head-on. Within hours of the patients falling ill, hospital staff identified the contaminated contrast agent as the likely source, halting further use of the implicated batch. Of the eight affected individuals, three required intensive care due to the severity of their reactions, though they have since been transferred to regular wards for continued monitoring. Two others remain hospitalized, while three were discharged shortly after initial treatment. Acting General Manager Peter Van Puyvelde publicly expressed profound regret over the incident, emphasizing that the well-being of patients remains the top priority. The hospital mobilized internal teams to trace the contamination, working tirelessly to uncover how such a failure occurred in a setting expected to uphold the highest safety standards.
Transparency has been a cornerstone of the hospital’s approach in the wake of this troubling event. Regular updates have been provided to the public and affected families, ensuring that trust is maintained despite the uncertainty surrounding the contamination’s origin. Van Puyvelde highlighted the exhaustive efforts by staff to review every step of the process, from storage to administration, in an attempt to pinpoint where the breach occurred. While no definitive answers have emerged yet, this openness has helped mitigate some of the immediate backlash, showing a dedication to accountability. The focus on patient recovery, coupled with a proactive stance on communication, sets a precedent for how medical institutions can handle such crises, even as the full scope of the incident continues to unfold under intense scrutiny.
Patient Impact and Recovery Progress
The human toll of the incident has been significant, with each of the eight patients experiencing varying degrees of illness due to the contaminated contrast agent. Initial symptoms ranged from fever and chills to more severe systemic reactions, necessitating urgent medical intervention for several individuals. The three patients who required intensive care faced the most critical conditions, battling complications that could have long-term implications for their health. Fortunately, their transfer to regular wards signals a positive step in their recovery journey, though medical teams remain vigilant for any lingering effects of the bacterial exposure. This situation has shed light on the fragility of patient safety during routine procedures like CT scans, which are typically considered low-risk.
Beyond the immediate health concerns, the emotional and psychological impact on the affected patients and their families cannot be overlooked. Hospitalization, even for those discharged, often brings stress and uncertainty, compounded by the knowledge that a preventable error led to their suffering. AZ Sint-Blasius Hospital has prioritized support services, offering counseling and regular updates to ease concerns during this difficult period. Meanwhile, the disparity in recovery timelines—some patients returning home while others remain under care—underscores the unpredictable nature of infections caused by bacteria like Klebsiella. This incident serves as a stark reminder of the need for robust safeguards to protect patients from unforeseen risks in medical settings, pushing the conversation toward systemic improvements.
Investigation Challenges and Hypotheses
Exploring Possible Causes
Unraveling the mystery behind the contamination of the Iomeron 400 contrast agent has proven to be a complex endeavor for both hospital officials and external investigators. Several potential sources have been hypothesized, spanning the entire supply chain from manufacturing to administration. One theory points to a possible issue at the supplier level, suggesting that the contamination may have originated during production or packaging before reaching the hospital. Another consideration involves improper storage conditions at AZ Sint-Blasius, where temperature fluctuations or environmental factors could have compromised the integrity of the contrast agent. Each of these possibilities carries significant implications for how medical supplies are managed across the industry, highlighting the need for stringent oversight at every stage.
Additionally, attention has turned to the administration process itself as a potential point of failure in this troubling incident. Errors during the handling of the contrast agent, such as inadequate sterilization practices or cross-contamination during preparation, remain under scrutiny as viable explanations. The CT injector system used during the scans has also been flagged as a possible conduit for the bacteria, raising questions about equipment maintenance and hygiene protocols. With the incident confined to a narrow two-hour window affecting eight consecutive patients, the focus on procedural missteps has intensified. Both the hospital and the manufacturer, Bracco, continue to probe these theories, but the absence of a definitive answer underscores the intricate nature of tracing contamination in a high-stakes medical environment.
Broader Implications for Radiology Safety
The ongoing investigation into this incident reveals deeper challenges within radiology departments that extend beyond a single hospital or batch of contrast agent. The uncertainty surrounding the contamination’s origin points to systemic gaps in infection control protocols, which, if left unaddressed, could lead to similar events elsewhere. Experts have noted that while such occurrences are rare, the potential for bacterial contamination in medical substances like contrast agents is an ever-present risk when protocols are not rigorously enforced. This situation calls for a comprehensive review of current practices, from how multidose vials are managed to the frequency of equipment sterilization, to ensure that vulnerabilities are minimized across the board.
Moreover, the collaborative nature of the investigation—involving hospital staff, manufacturer representatives, and regulatory authorities—highlights the importance of shared responsibility in safeguarding patient health. Belgian authorities have taken precautionary measures by quarantining samples from the implicated batch, a step that could inform future responses to similar crises. The emphasis on pinpointing whether the issue lies in supply, storage, or administration reflects a broader push for accountability and transparency in healthcare. As findings emerge, they are likely to influence updated guidelines and training programs aimed at preventing contamination, ensuring that radiology departments are better equipped to handle the complexities of modern medical procedures with the highest safety standards in mind.
Understanding Klebsiella and Its Risks
Bacterial Threat in Medical Settings
Klebsiella bacteria, the identified contaminant in the Iomeron 400 contrast agent, typically resides harmlessly in the human gut but can become a formidable threat under certain conditions, particularly in hospital environments. For healthy individuals, this bacterium poses little risk, as the body’s natural defenses are often sufficient to keep it in check. However, for vulnerable patients—those with compromised immune systems, recent surgeries, or chronic health conditions—Klebsiella can trigger severe infections, ranging from pneumonia to bloodstream infections. This incident at AZ Sint-Blasius Hospital exemplifies how quickly such a pathogen can exploit weaknesses in medical settings, especially when introduced through invasive procedures like CT scans involving contrast agents.
The transmission of Klebsiella often occurs through direct contact, whether via contaminated equipment, unsterilized hands, or improperly handled medical substances, making hygiene protocols paramount in preventing outbreaks. Hospitals are known hotspots for this bacterium due to the high concentration of at-risk patients and the frequent use of shared equipment. The incident on August 27 serves as a critical reminder of the stakes involved, where even a single lapse in sterility can have cascading effects on multiple individuals. As investigations continue, the focus on reinforcing strict procedural standards and staff training becomes essential to curb the risks posed by such opportunistic pathogens in clinical environments.
Vulnerability of Hospitalized Patients
The specific dangers Klebsiella presents to hospitalized patients underscore why this incident has garnered such intense attention from medical professionals and regulators alike. Patients undergoing procedures like CT scans often already face health challenges, whether due to underlying illnesses or the stress of recent medical interventions, leaving them particularly susceptible to infections. When introduced through a contaminated contrast agent, as seen in this case, the bacteria can rapidly spread within the body, leading to severe complications that require aggressive treatment. The fact that three of the eight affected patients needed intensive care highlights the potential lethality of such infections when safeguards fail.
Beyond the physical risks, the prevalence of Klebsiella in hospital settings adds a layer of complexity to infection control efforts, as it can persist on surfaces and equipment if not properly addressed. Routine procedures, often perceived as safe, become potential vectors for harm when contamination occurs, eroding trust in medical systems. This event emphasizes the urgent need for enhanced vigilance, including regular audits of hygiene practices and the adoption of single-use materials where feasible. Protecting the most vulnerable requires a multifaceted approach, ensuring that every aspect of patient care—from equipment to staff protocols—prioritizes the elimination of preventable risks like bacterial exposure.
Expert Insights and Historical Context
Lessons from the Past and Current Recommendations
Drawing from historical precedents, the incident at AZ Sint-Blasius Hospital echoes past tragedies that have shaped modern infection control debates, such as a devastating 2001 case in Germany where contaminated contrast media led to fatal infections. These earlier events revealed the catastrophic potential of procedural oversights, particularly with multidose vials that can spread contamination across multiple patients if mishandled. Experts reviewing the current situation have pointed to similar risks, advocating for a shift toward single-dose vials to minimize cross-contamination opportunities. Additionally, recommendations include stricter temperature controls for storage and enhanced supervision during the administration of contrast agents to prevent breaches in sterility.
Current insights from professionals like Dr. Francisco Vega from Hospital Universitario de la Princesa in Madrid further illuminate the likely scenario behind the contamination. Dr. Vega suggests that a breach in the infusion system’s sterility, combined with the use of a multidose vial, likely facilitated the spread of Klebsiella among the eight patients. This perspective aligns with the bacterium’s known prevalence in hospital settings, where it often contributes to healthcare-associated infections. The call for updated protocols, including regular equipment audits and comprehensive staff training, emerges as a critical response to such incidents. These recommendations aim to fortify radiology practices against rare but severe risks, ensuring patient safety remains paramount in every procedure.
Evolving Standards in Infection Control
The broader implications of expert analyses extend to how infection control standards must evolve to keep pace with the complexities of modern healthcare delivery. While contamination events tied to contrast agents are uncommon, their impact—as evidenced by the severe reactions of the affected patients—demands a reevaluation of existing safeguards. Specialists argue that the rarity of such incidents should not breed complacency but instead inspire proactive measures, such as adopting advanced sterilization technologies and revising guidelines for handling medical substances. The historical context of bacterial outbreaks in hospitals, often linked to contaminated instruments or environmental sources, reinforces the need for a holistic approach to prevention that addresses every potential vulnerability.
Moreover, the dialogue sparked by this incident highlights a pressing need for international collaboration among healthcare providers to share best practices and lessons learned from past failures. The focus on single-use materials over cost-effective multidose options represents a shift toward prioritizing safety, even at the expense of efficiency. As radiology departments grapple with balancing operational demands and patient well-being, the push for stricter oversight and continuous improvement in infection control practices becomes undeniable. This evolving landscape of standards seeks to ensure that tragic events like the one on August 27 become relics of the past, replaced by a fortified commitment to safeguarding health at every turn.
Industry and Regulatory Actions
Collaborative Efforts for Patient Safety
In response to the contamination incident, Bracco, the manufacturer of Iomeron 400, has initiated a comprehensive investigation into their production and distribution processes to determine if any lapses occurred on their end. Preliminary findings suggest no quality issues within their manufacturing facilities, pointing to the possibility that the contamination was introduced at a later stage, potentially during storage or handling at the hospital. This isolated incident, confined to a specific batch used on August 27, has prompted close collaboration between Bracco and AZ Sint-Blasius Hospital to trace the root cause. Their joint efforts reflect a shared commitment to patient safety, aiming to uncover actionable insights that can prevent similar occurrences in the future across the medical field.
Belgian regulatory authorities have also stepped in with decisive action, ordering the quarantine of samples from the implicated batch as a precautionary measure while investigations unfold. This move underscores the seriousness with which such incidents are treated, ensuring that potentially compromised materials are removed from circulation until cleared. The regulatory oversight adds a critical layer of accountability, pushing for transparency from all involved parties. As Bracco continues to scrutinize their logistics chain, the industry-wide emphasis on rigorous quality control and rapid response mechanisms becomes evident, setting a standard for how manufacturers and regulators can work together to uphold trust in medical products.
Future Safeguards and Industry Trends
The ripple effects of this incident are likely to influence long-term changes in how contrast agents and other medical substances are managed within the healthcare sector. Industry trends are increasingly leaning toward enhanced safety measures, such as the adoption of single-use vials to eliminate the risk of cross-contamination inherent in multidose containers. Additionally, there is a growing call for advanced tracking systems to monitor the journey of medical products from production to patient administration, ensuring any anomalies are detected early. These innovations, while potentially increasing costs, prioritize the prevention of rare but devastating events like the one experienced by the eight patients in Belgium.
Furthermore, the collaborative framework established by this case—bringing together manufacturers, hospitals, and regulatory bodies—sets a precedent for future crisis management in healthcare. The focus on shared responsibility encourages the development of universal guidelines that can be adopted globally, reducing the likelihood of contamination through standardized best practices. As investigations conclude, the findings are expected to inform policy updates and training programs, equipping medical professionals with the tools needed to navigate the complexities of infection prevention. This forward-looking approach aims to transform a tragic incident into a catalyst for systemic improvement, ensuring safer medical environments for all patients.