The fight against advanced prostate cancer often reaches a critical juncture when tumors become resistant to standard hormone therapies, leaving patients with limited and often challenging treatment avenues. In a landmark development, a new clinical trial initiated at The James Cook University Hospital in Middlesbrough is now offering a beacon of hope, making its patients among the first in the United Kingdom to access a highly targeted experimental therapy. The study centers on Lutetium (177Lu) rhPSMA-10.1, a cutting-edge radioactive injection designed for men whose cancer has progressed despite hormonal treatments. This innovative approach represents a significant step forward in personalized medicine, aiming to attack cancerous cells directly while preserving the integrity of surrounding healthy tissues. The trial’s launch marks a pivotal moment for those facing one of the most common cancers in men, promising to explore new strategies for individuals who have exhausted conventional options and are in dire need of a breakthrough.
A New Frontier in Precision Oncology
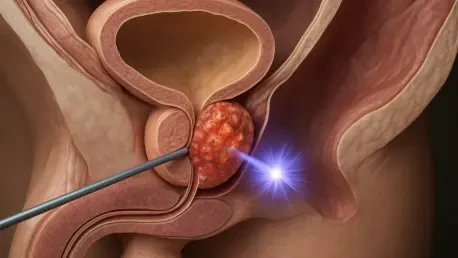
At the core of this pioneering trial is a sophisticated form of radiopharmaceutical therapy, a treatment modality that functions like a “smart bomb” for cancer. The therapy, Lutetium (177Lu) rhPSMA-10.1, is engineered to seek out and bind to a protein called Prostate-Specific Membrane Antigen (PSMA), which is found in high concentrations on the surface of prostate cancer cells. Once attached, the radioactive isotope Lutetium-177 delivers a highly localized dose of radiation, destroying the cancer cells from within. According to Dr. Darren Leaning, a consultant clinical oncologist at the hospital, this novel version of the therapy has been specifically developed by Blue Earth Therapeutics to deliver its radioactive payload with maximum efficiency. While similar targeted radiotherapies have already demonstrated effectiveness, this advanced formulation is designed to improve upon existing options, potentially offering a more potent and precise attack on the disease while minimizing collateral damage to healthy organs and tissues.
Refining Treatment Through Rigorous Study
The primary objectives of this Phase 2 trial went beyond simply confirming the therapy’s efficacy; researchers sought to meticulously define its clinical application. A key focus was on establishing the treatment’s safety profile and determining the optimal dosing schedules to maximize patient outcomes. Investigators explored various administration plans, including the possibility of administering higher or more frequent doses, to evaluate whether such adjustments could lead to improved responses. Furthermore, the study was designed to provide crucial data on the drug’s pharmacokinetics—how it is absorbed, distributed, and metabolized within both cancerous and healthy cells. This detailed understanding helped physicians better assess its impact on reducing measurable disease and, importantly, on enhancing patients’ quality of life. Ultimately, the trial represented a significant step in the ongoing effort to advance care for prostate cancer, with the long-term goal of solidifying radiopharmaceutical therapies as a foundational treatment option for a wider variety of cancers.