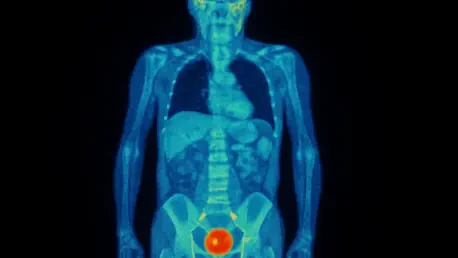
The advent of targeted radiopharmaceutical therapies like (177Lu)Lu-PSMA-617 has offered a significant new weapon in the fight against metastatic castration-resistant prostate cancer (mCRPC), but predicting which patients will benefit most versus those who will suffer severe side effects remains a critical challenge. Prostate-specific membrane antigen (PSMA) positron emission tomography (PET) imaging stands at the forefront of this effort, providing a detailed map of cancer spread and intensity that clinicians hope to use to tailor treatments. A recent investigation, however, has unveiled a perplexing and counterintuitive relationship between PSMA PET metrics and treatment-related toxicity. The study, designed to bring clarity, has instead presented researchers with a sophisticated paradox, suggesting that the very indicators of a high tumor burden might also predict both a faster and a slower onset of adverse hematologic events, complicating the path toward personalized medicine in this complex disease. This finding underscores the intricate biological interplay at work and highlights the urgent need for a deeper understanding of these advanced imaging biomarkers.
A Complex Correlation
In a retrospective analysis of 61 patients with mCRPC, researchers leveraged sophisticated artificial intelligence software to meticulously quantify baseline PSMA PET parameters before the administration of (177Lu)Lu-PSMA-617 therapy. The goal was to identify predictive markers for hematologic toxicity, a common and serious side effect of the treatment. The software calculated several key metrics, including the prostate tumor volume, the mean standardized uptake value (SUVmean), which measures the intensity of PSMA expression, and the total lesion PSMA (TLP), a composite metric that multiplies tumor volume by SUVmean. The analysis produced a set of seemingly contradictory results centered on bone metastases. The first finding indicated that a higher total lesion PSMA in bone was correlated with a 31% greater risk of experiencing an earlier onset of severe toxicity. However, a separate multivariable analysis isolated a different trend: pronounced PSMA expression within bone metastases, as measured by SUVmean, was significantly associated with a delayed onset of these same severe toxicities. This creates a clinical conundrum where one aspect of high tumor activity appears to accelerate harm while another seems to protect against it.
Reconciling Contradictory Findings
The study’s findings prompted a call for cautious interpretation, particularly given the relatively small cohort of patients involved. Study co-author Dr. Jeremie Calais emphasized the need for careful consideration, questioning the biological clarity of the composite TLP metric and expressing a preference for assessing SUVmean and tumor volume as separate, more distinct variables. To reconcile the paradoxical results, a compelling hypothesis emerged that centered on the mechanism of the therapy itself. It was proposed that higher PSMA expression might more effectively draw the radiopharmaceutical agent directly to the cancerous cells within the bone. This targeted delivery could lead to more efficient tumor killing, which in turn might create space and reduce the systemic stress that allows healthy bone marrow to recover and regrow. This regenerative process could ultimately delay the onset of hematologic toxicity. This line of reasoning suggested that while a large tumor volume (a component of high TLP) represents a greater overall disease burden that could lead to faster toxicity, high PSMA expression within that tumor could signify a stronger therapeutic response that ultimately had a protective effect. This complex relationship underscored that a simple reading of imaging data is insufficient and that future progress will depend on larger studies to validate these initial findings and untangle these intricate biological signals.