
In a world where medical technology evolves at an unprecedented pace, ensuring safety and reliability through standardized practices has never been more critical, and one individual stands at the forefront of this mission with groundbreaking contributions that are shaping the future of healthcare.
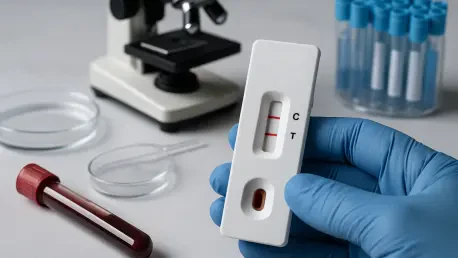
In the ever-evolving landscape of global healthcare, the In Vitro Diagnostic (IVD) Test Market stands as a critical pillar, providing essential tools for detecting diseases, monitoring health conditions, and informing treatment strategies. These tests, conducted outside the human body using samples
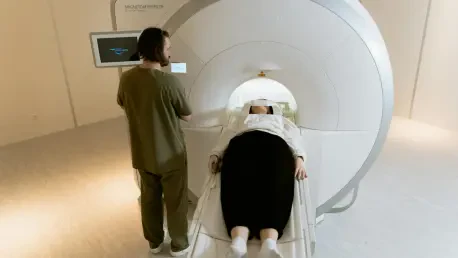
In a medical controversy that has gripped public attention, the recent MRI scan of former President Donald Trump at Walter Reed Medical Center has ignited intense discussion about transparency and the state of his health, raising critical questions about the credibility of official information.
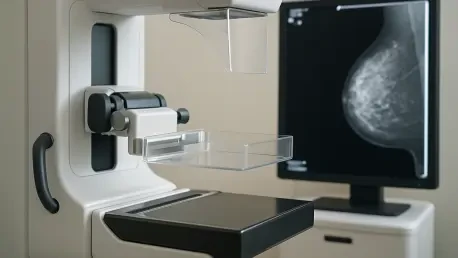
In the ever-evolving landscape of breast cancer screening, a transformative force is emerging through artificial intelligence (AI), poised to redefine how mammographic breast density is assessed. For decades, radiologists have relied on subjective visual evaluations to categorize breast density, a
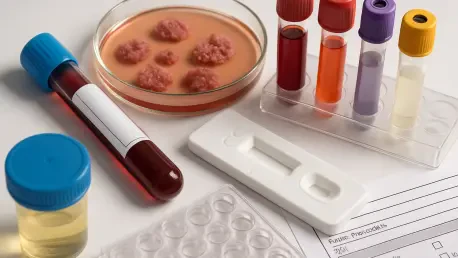
In a groundbreaking stride for medical innovation, BCAL Diagnostics, an Australian company listed on the ASX under the ticker BDX, has emerged as a beacon of hope in the fight against cancer with its latest diagnostic advancements. The introduction of cutting-edge blood tests for pancreatic and
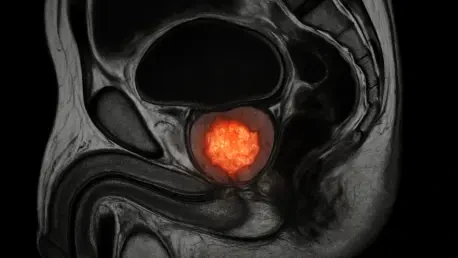
In a landscape where prostate cancer remains one of the most common cancers among men worldwide, the search for efficient, accessible, and accurate diagnostic tools has never been more critical, especially as recent advancements bring a new contender—micro-ultrasound technology—to the forefront.