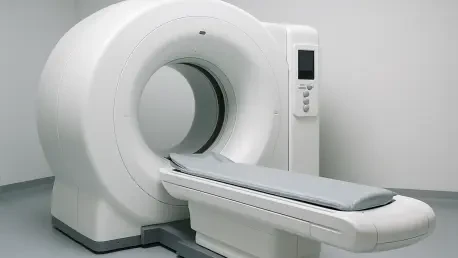
Imagine a world where neurodegenerative diseases are detected at their earliest stages, long before symptoms become debilitating, allowing for interventions that can dramatically alter a patient’s life trajectory. This vision is inching closer to reality with a groundbreaking advancement in medical imaging technology from Brightonix Imaging. The company recently achieved a significant milestone with the FDA clearance of its flagship product, the PHAROS PET Scanner, for commercial distribution across the United States. This clinical positron emission tomography (PET) system promises to redefine nuclear imaging by delivering unparalleled image quality, aiding healthcare providers in early disease detection, precise diagnosis, and personalized treatment planning, especially in fields like neurology. As the medical community anticipates its widespread adoption, the potential for this technology to reshape diagnostic standards and improve patient outcomes is generating considerable excitement. This development marks a pivotal moment in the ongoing evolution of precision medicine.
Cutting-Edge Features Redefining Imaging Standards
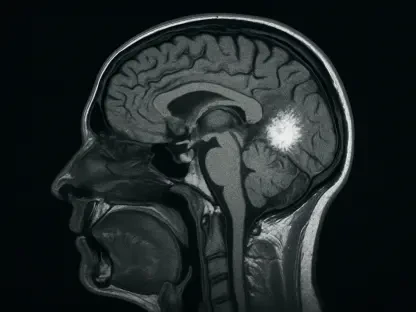
The PHAROS PET Scanner stands out in the crowded field of medical imaging due to its innovative design and advanced capabilities. At the heart of this system is state-of-the-art detector technology that produces exceptional image clarity, enabling clinicians to spot even the most subtle anomalies with remarkable accuracy. This level of detail is crucial for early detection of conditions such as Alzheimer’s or Parkinson’s, where minute changes in brain tissue can signal the onset of disease. Beyond its technical prowess, the scanner’s compact footprint addresses a practical challenge faced by many healthcare facilities. Designed to fit into spaces with limited room, it ensures that high-quality imaging is accessible to a broader range of clinical settings, from large hospitals to smaller imaging centers. Such adaptability makes it a game-changer for institutions striving to upgrade their diagnostic tools without requiring extensive infrastructure changes, thereby democratizing access to cutting-edge technology.
Another standout aspect of the PHAROS PET Scanner is its versatility in clinical applications, paired with a focus on patient comfort. The system offers configurable setups tailored for specific imaging needs, including brain, breast, and extremity scans, ensuring flexibility for diverse diagnostic scenarios. Additionally, it accommodates various patient positions, whether seated or lying down, which significantly enhances the experience for individuals undergoing scans. This thoughtful design not only prioritizes comfort but also improves compliance, as patients are more likely to complete necessary imaging without distress. Complementing these features is a user-friendly interface that streamlines workflows for clinicians and technologists. By reducing the learning curve and minimizing operational complexities, the scanner integrates seamlessly into busy medical practices, allowing staff to focus on patient care rather than grappling with cumbersome technology. This combination of functionality and ease of use positions the system as a transformative tool in modern diagnostics.
Impact on Patient Outcomes and Precision Medicine
The potential of the PHAROS PET Scanner to improve patient outcomes cannot be overstated, particularly in the realm of neurodegenerative diseases. Early and accurate diagnosis is often the key to effective management of such conditions, and this technology empowers healthcare providers to identify issues at their inception. With its high-resolution imaging, the scanner enables detailed visualization of brain structures, facilitating precise assessments that can guide tailored treatment plans. This capability aligns with the growing emphasis on precision medicine, where diagnostic tools are expected to provide individualized insights rather than one-size-fits-all solutions. By offering a clearer window into complex conditions, the system supports clinicians in making informed decisions that can delay disease progression or improve quality of life, marking a significant leap forward in patient-centered care within neurology and beyond.
Beyond its diagnostic precision, the PHAROS PET Scanner reflects a broader trend of collaboration and innovation in healthcare technology. Supported by initiatives like the Korea Medical Device Development Fund (KMDF), its development underscores the value of partnerships between industry and government in pushing the boundaries of medical advancements. This collaborative spirit ensures that cutting-edge tools are not only conceptualized but also brought to market, addressing real-world clinical needs. The scanner’s FDA clearance further validates its reliability and efficacy, instilling confidence among healthcare providers who seek dependable solutions for their patients. As the medical field continues to evolve, technologies like this one serve as a reminder of how targeted investments and shared goals can yield innovations that tackle pressing health challenges, ultimately benefiting society by enhancing the accuracy and accessibility of critical diagnostic services.
A New Era for Clinical Diagnostics
Reflecting on the journey of the PHAROS PET Scanner, its FDA clearance stood as a landmark achievement that validated years of research and development by Brightonix Imaging. The technology’s rollout across the United States marked the beginning of a transformative phase in nuclear imaging, where high-resolution capabilities and practical design converged to elevate clinical standards. Its impact was evident in the way it empowered healthcare providers to detect and manage conditions with unprecedented precision, particularly in neurology.
Looking ahead, the focus should shift to ensuring widespread adoption and training for medical professionals to maximize the scanner’s potential. Healthcare institutions are encouraged to explore integration strategies that prioritize accessibility, while ongoing research could further refine its applications. As the industry moves forward, fostering dialogue between innovators, clinicians, and policymakers will be essential to address emerging needs and sustain momentum in advancing diagnostic technologies for future generations.