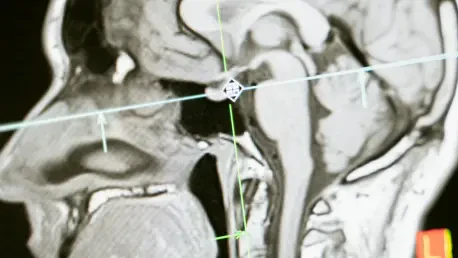
A groundbreaking artificial intelligence algorithm is providing an unprecedented window into the brainstem, one of the most complex and historically inaccessible regions of the human brain, which could fundamentally change how neurological disorders are diagnosed and treated. Developed through a landmark collaboration between researchers at MIT, Harvard University, and Massachusetts General Hospital, this publicly available software, known as the BrainStem Bundle Tool (BSBT), utilizes diffusion MRI scans to automatically map out eight critical white matter pathways with stunning detail. This significant advancement is set to revolutionize neuroscience by enabling the creation of novel biomarkers for a range of devastating conditions and offering, for the first time, a quantifiable way to track patient recovery from severe brain injuries. The brainstem serves as the body’s essential command center, orchestrating fundamental functions like consciousness, breathing, heart rate, and the sleep-wake cycle. These vital signals are transmitted through densely packed bundles of nerve fibers known as white matter. However, the brainstem’s small size, combined with imaging distortions caused by breathing and blood flow, has long rendered it a “blind spot” for conventional imaging techniques. This limitation has severely hampered the ability of both researchers and clinicians to understand how diseases and traumatic injuries impact these crucial neural highways, leaving a significant gap in modern medicine.
Forging a New Lens on the Brain’s Core
The development of the BSBT algorithm hinged on overcoming long-standing imaging challenges through the sophisticated application of artificial intelligence, specifically a type of AI model called a “convolutional neural network” (CNN). This advanced network was designed to analyze data from diffusion MRI, a specialized imaging technique that traces the movement of water molecules as they travel along the long, myelinated branches of neurons, known as axons. The process began by first generating “probabilistic fiber maps,” which outlined the likely trajectories of the major white matter pathways descending into the brainstem from higher brain regions like the thalamus and cerebellum. The CNN then integrated these probabilistic maps with multiple channels of direct imaging information sourced from within the brainstem itself. By expertly combining these disparate data sources, the network learned to accurately distinguish and segment eight individual fiber bundles, a task that was previously impossible to automate with such precision. This intricate, multi-stage approach equipped the AI with the nuanced capability to identify these critical structures automatically, effectively creating a high-definition roadmap of the brainstem’s internal wiring from standard clinical scans.
To ensure the algorithm was not only innovative but also clinically reliable, the research team subjected BSBT to a series of rigorous validation tests. The AI was initially trained using a set of 30 meticulously, manually annotated diffusion MRI scans from the Human Connectome Project, a large-scale effort to map the human brain’s connections. The true test of its accuracy, however, came from comparing its automated segmentations against “ground truth” data obtained from the painstaking dissection of post-mortem human brains. In these anatomical studies, the neural bundles could be clearly delineated through microscopic inspection or imaged with ultra-high-resolution scanners, providing an unequivocal benchmark for the algorithm’s performance. Beyond this anatomical validation, the team confirmed the tool’s consistency by analyzing scans from 40 healthy volunteers taken two months apart, demonstrating that BSBT could reliably identify the exact same bundles in both scans for each individual. The researchers even performed a component analysis, systematically disabling parts of the neural network to verify that each element was contributing meaningfully to the final, accurate output, thus establishing the tool’s robust and dependable nature.
From Algorithm to Clinical Insight
With its accuracy firmly established, the BSBT was applied to extensive datasets of diffusion MRI scans from patients with a range of neurological conditions, marking a pivotal transition from a research tool to a potential clinical powerhouse. The algorithm was used to measure two key metrics: the volume of each white matter bundle and its “fractional anisotropy” (FA), a value that reflects the structural integrity of the nerve fibers. High FA values indicate healthy, well-organized axons, while a reduction suggests damage, degradation, or disease. The study yielded several remarkable findings that demonstrated distinct patterns of structural change specific to each condition. For instance, in patients with Parkinson’s disease, BSBT revealed a significant reduction in FA in three of the eight identified bundles and detected a loss of volume in another bundle when comparing baseline scans to follow-ups conducted two years later. In contrast, patients with multiple sclerosis exhibited the most pronounced FA reductions across four specific bundles, along with volume loss in three. These detailed, quantitative assessments provide a powerful adjunct to current diagnostic imaging, offering structural and longitudinal information that was previously inaccessible and paving the way for more precise diagnoses.
The tool’s potential for forecasting patient outcomes and monitoring recovery was brought into sharp focus through the compelling case of a 29-year-old man recovering from a severe traumatic brain injury that had left him in a coma. By analyzing a series of scans taken over the seven-month period of his recovery, the BSBT algorithm revealed that his brainstem bundles had been physically displaced by the injury rather than severed. The software was able to quantify a threefold decrease in the volume of the lesions affecting these bundles over time, meticulously tracking their physical healing and their movement back into their proper anatomical positions. This observed structural recovery mirrored the patient’s own remarkable journey back to consciousness. This powerful demonstration suggests that the BSBT could have substantial prognostic potential, allowing clinicians to identify preserved brainstem pathways that may facilitate coma recovery. By providing such a detailed view into the brainstem’s healing process, the tool offers more than just a diagnosis; it provides a dynamic picture of the brain’s capacity for repair, opening a new frontier in the management of severe brain injuries.
The Dawn of a New Neurological Era
The successful development and validation of the BSBT represented more than just an incremental improvement in neuroimaging; it marked the beginning of a new chapter in the study of the human brain. By providing a reliable method to visualize and quantify the brainstem’s white matter, the tool has given researchers and clinicians unprecedented access to the core systems that regulate fundamental physiology. This newfound capability allowed for a deeper understanding of how neurodegenerative diseases and traumatic injuries manifest at a microstructural level. The distinct patterns of bundle degradation identified in conditions like Parkinson’s disease, multiple sclerosis, and traumatic brain injury suggested that these disorders could one day be differentiated and staged with far greater accuracy. This new “window” into the brainstem promised not only to refine diagnostics but also to accelerate progress in understanding the intricate mechanics of consciousness, motor control, and autonomic function, which have long been shrouded in mystery due to technical limitations. The work done by the collaborative team laid a critical foundation for future explorations into the brain’s most vital and enigmatic region, shifting the paradigm from broad observation to precise, data-driven analysis.