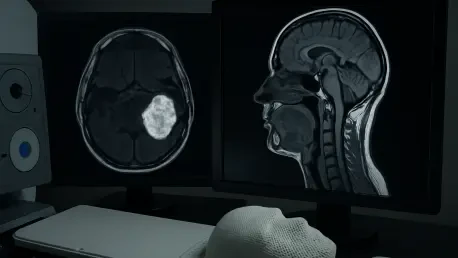
Imagine a scenario where cancer treatment becomes so finely tuned that it zeroes in on the most aggressive tumor regions while safeguarding delicate surrounding tissues, offering hope to those battling complex cancers like head and neck squamous cell carcinoma (HNSCC). For individuals diagnosed with HNSCC, a particularly challenging form of cancer due to its proximity to vital structures like salivary glands and vocal cords, this vision is inching closer to reality with the advent of diffusion-weighted magnetic resonance imaging (DW-MRI). This cutting-edge imaging technology provides a detailed glimpse into the tumor’s internal dynamics, potentially transforming the landscape of radiation therapy. Unlike traditional methods that often apply uniform doses, DW-MRI enables a more targeted approach, which could redefine treatment precision. A recent study published in a prominent medical journal has sparked significant interest by comparing DW-MRI-guided dose-painting intensity-modulated radiation therapy (DP-IMRT) with conventional MRI-based IMRT, shedding light on its potential to enhance outcomes while minimizing harm. This exploration aims to unpack the study’s findings and their broader implications for personalized cancer care.
Challenges in Treating Head and Neck Cancer
Navigating the Complexity of HNSCC
Head and neck squamous cell carcinoma presents a formidable challenge in oncology, primarily due to its location near critical anatomical structures that are highly sensitive to radiation. The proximity to organs like the larynx, pharynx, and salivary glands means that even slight miscalculations in treatment can lead to profound side effects, such as impaired speech or difficulty swallowing. These consequences often diminish patients’ quality of life long after the cancer is addressed. Traditional radiation approaches, while effective to an extent, struggle to differentiate between varying levels of tumor aggression and the surrounding healthy tissue. This limitation underscores the urgent need for more precise technologies that can adapt to the unique demands of each case, ensuring that therapeutic efforts focus squarely on malignant cells without collateral damage to essential functions.
The complexity of HNSCC treatment extends beyond anatomy to the tumor’s biological behavior, which can vary widely even within a single patient. Some tumor regions may exhibit greater resistance to radiation, requiring higher doses for effective control, while other areas might respond to standard levels. Without tools to map these differences, clinicians often face a dilemmescalate doses and risk toxicity, or maintain safer levels and potentially miss aggressive subvolumes. The introduction of advanced imaging like DW-MRI offers a promising avenue to address this conundrum by providing insights into the tumor’s microenvironment. Such technology could guide radiation planning with unprecedented detail, potentially reducing the incidence of severe side effects while improving control over the disease, marking a significant step forward in managing this intricate cancer type.
Shortcomings of Standard Radiation Methods
Conventional MRI-based intensity-modulated radiation therapy (IMRT) represents a significant advancement over older techniques, yet it falls short in fully addressing the nuanced needs of HNSCC patients. This method typically delivers a uniform radiation dose across the entire tumor volume, based on anatomical imaging that highlights size and location rather than internal characteristics. As a result, areas within the tumor that are more aggressive or resistant to treatment may not receive the necessary intensity to eradicate them, increasing the risk of recurrence. Simultaneously, healthy tissues adjacent to the tumor often endure unnecessary exposure, leading to side effects that can be debilitating over time, such as chronic dry mouth or swallowing difficulties.
Moreover, the lack of functional data in conventional IMRT planning limits its ability to adapt to the dynamic nature of tumors, which can change during the course of treatment. Without real-time insights into cellular density or radioresistance, adjustments to the radiation plan remain largely reactive rather than proactive. This gap in precision highlights the potential value of integrating more sophisticated imaging modalities that can reveal hidden tumor traits. By failing to account for intratumoral heterogeneity, standard IMRT may not fully optimize outcomes, paving the way for newer approaches like DW-MRI-guided therapy to fill this critical void and offer a more tailored solution for patients facing this challenging diagnosis.
The Role of DW-MRI in Enhancing Radiation Therapy
Unveiling Tumor Dynamics with Advanced Imaging
Diffusion-weighted magnetic resonance imaging stands as a transformative tool in oncology by capturing the subtle movement of water molecules within tissues, offering a window into the tumor’s internal structure that conventional imaging cannot match. This functional perspective reveals variations in cellular density and tissue composition, pinpointing areas of potential radioresistance or heightened aggression within the tumor. For HNSCC, where such differences can significantly impact treatment success, DW-MRI provides critical data that can inform more effective radiation strategies. By mapping these unique characteristics, clinicians gain the ability to focus therapeutic efforts on the most problematic zones, potentially improving control over the cancer while reducing unintended harm to surrounding healthy structures.
Beyond its diagnostic capabilities, DW-MRI serves as a cornerstone for adapting treatment plans to the specific needs of each patient, moving away from generalized protocols. The detailed insights it offers enable a deeper understanding of how different tumor regions might respond to radiation, allowing for a more strategic allocation of doses. This approach contrasts sharply with traditional methods that rely solely on anatomical boundaries, often overlooking the biological intricacies at play. As a result, DW-MRI not only enhances the precision of radiation delivery but also supports a shift toward more individualized care, addressing the unique challenges posed by HNSCC and setting a new benchmark for how complex cancers are managed in clinical settings.
Precision Targeting through Dose Painting
The concept of dose painting, enabled by DW-MRI, marks a significant evolution in radiation therapy by allowing clinicians to vary radiation intensity based on the tumor’s biological profile rather than treating it as a uniform mass. This technique involves escalating doses to specific subvolumes identified as high-risk or resistant, ensuring that these areas receive the attention needed to maximize tumor control. For patients with HNSCC, where aggressive tumor pockets can lurk amidst less threatening regions, dose painting offers a way to tackle the cancer with surgical precision. By sparing adjacent healthy tissues from excessive radiation, this method aims to curb the severe side effects that often accompany treatment, preserving critical functions like speech and swallowing.
Implementing dose painting requires a sophisticated integration of imaging data into radiation planning systems, ensuring that the escalated doses are delivered accurately to the intended targets. This precision is particularly vital in the head and neck region, where even minor deviations can impact vital structures. The ability to customize radiation in this manner represents a departure from the broader, less discriminating approaches of the past, highlighting a pathway to better outcomes. As dose painting gains traction, supported by technologies like DW-MRI, it could redefine standards of care, offering a more effective and safer option for managing complex cancers, ultimately benefiting patient recovery and long-term well-being.
Insights from Recent Research on DW-MRI
Significant Gains in Disease Management
A groundbreaking study by Tan et al., published in a leading medical journal, has provided compelling evidence of the advantages offered by DW-MRI-guided DP-IMRT over conventional MRI-based IMRT in treating HNSCC. The research, involving a substantial cohort of patients, demonstrated that those treated with the DW-MRI approach achieved a two-year disease-free survival rate of 75.2%, notably higher than the 63.8% observed in the standard IMRT group. This improvement suggests that identifying and targeting specific tumor subvolumes with higher radiation doses can significantly enhance the ability to prevent cancer progression. Such findings hold promise for reducing the physical and emotional toll of repeated treatments, offering patients a better chance at sustained remission.
Additionally, the study highlighted a marked improvement in locoregional recurrence-free survival, with the DW-MRI group reaching 75.9% compared to 64.7% in the conventional group. This statistic underscores the potential of functional imaging to address local cancer return, a persistent challenge in HNSCC management. By focusing radiation on areas most likely to resist treatment, DW-MRI-guided therapy tackles a critical barrier to long-term success. These results not only validate the efficacy of precision targeting but also signal a shift in how radiation strategies might be designed, prioritizing control over recurrence as a key metric of therapeutic achievement in oncology.
Maintaining Safety Alongside Effectiveness
One of the most striking outcomes from the comparative study was the ability of DW-MRI-guided DP-IMRT to deliver higher radiation doses without a corresponding increase in severe side effects. Despite the escalated intensity targeted at specific tumor zones, the rates of acute and late toxicities in the DW-MRI group remained comparable to those in the standard IMRT cohort. This balance is crucial for HNSCC patients, where treatment often risks damaging vital functions, leading to lifelong challenges. The precision of dose painting appears to confine the intensified radiation to malignant areas, effectively shielding surrounding healthy tissues from harm.
This finding carries significant weight for clinical practice, as it challenges the assumption that higher doses inevitably lead to greater toxicity. Instead, it suggests that with the right tools, radiation therapy can be both potent and protective, addressing the dual goals of eradicating cancer and preserving quality of life. For patients, this means the possibility of undergoing aggressive treatment without the fear of debilitating consequences, a factor that can influence treatment decisions and adherence. As such, the safety profile of DW-MRI-guided therapy strengthens its case as a viable advancement, paving the way for broader acceptance in managing complex cancers like HNSCC.
Broader Impacts on Cancer Treatment
Embracing Individualized Therapeutic Strategies
The success of DW-MRI-guided DP-IMRT in the treatment of HNSCC reflects a larger movement within oncology toward personalized medicine, where treatments are tailored to the distinct characteristics of each tumor rather than relying on standardized protocols. This shift acknowledges that no two cancers are identical, even within the same diagnosis, and that accounting for biological variations can lead to better results. By leveraging functional imaging to guide radiation, clinicians can move beyond merely considering a tumor’s size or location, instead focusing on its internal behavior to optimize therapeutic impact. This approach promises to enhance effectiveness across a range of cancer types, redefining how care is delivered.
Furthermore, personalization through technologies like DW-MRI aligns with the growing emphasis on patient-centered care, where the goal is not just survival but thriving post-treatment. Tailored radiation plans that address specific tumor traits reduce the likelihood of over- or under-treatment, striking a balance that benefits both disease control and recovery. This trend toward customization could inspire similar innovations in other treatment modalities, fostering a more holistic and adaptive framework in oncology. As such, the adoption of individualized strategies marks a pivotal moment, potentially setting new standards that prioritize precision over uniformity in cancer management.
Enhancing Life After Treatment
A key advantage of DW-MRI-guided therapy lies in its potential to improve quality of life for HNSCC survivors, a group often burdened by the lasting effects of radiation on critical functions. By minimizing damage to structures like the vocal cords and salivary glands, this approach helps preserve abilities such as speaking and swallowing, which are integral to daily living. Patients who retain these functions post-treatment are better equipped to return to normal routines, experiencing less dependency and greater emotional well-being. This focus on functional preservation alongside cancer control addresses a vital aspect of survivorship that is frequently overlooked in traditional metrics.
The ripple effects of improved quality of life extend beyond the individual to healthcare systems and society at large, as reduced treatment-related complications can lower the need for ongoing medical interventions and support services. Patients who maintain better health after therapy are also more likely to remain active in their communities, contributing economically and socially. Thus, the benefits of DW-MRI-guided DP-IMRT resonate on multiple levels, offering not just a clinical advancement but a pathway to more fulfilling lives for those who have faced the daunting journey of cancer treatment, highlighting the profound human impact of precision medicine.
Looking Ahead: Barriers and Opportunities
Addressing Practical Hurdles in Adoption
While the potential of DW-MRI-guided DP-IMRT is undeniable, integrating this technology into routine clinical practice presents several challenges that must be navigated carefully. The approach demands access to advanced imaging equipment and sophisticated radiation planning systems, resources that may not be readily available in all medical centers, particularly in under-resourced regions. Additionally, the expertise required to interpret DW-MRI data and translate it into effective treatment plans necessitates specialized training for radiologists, oncologists, and medical physicists. Without standardized protocols, inconsistencies in application could undermine the benefits observed in controlled studies, posing a barrier to widespread implementation.
Collaboration across medical disciplines emerges as a critical factor in overcoming these obstacles, ensuring that the technology is applied with precision and reliability. Establishing guidelines for imaging acquisition, data integration, and quality assurance will be essential to maintain consistency across diverse healthcare settings. Furthermore, investment in infrastructure and education can help bridge gaps in access, enabling more facilities to adopt this promising approach. Tackling these practical hurdles is not merely a logistical necessity but a step toward equity in cancer care, ensuring that advancements like DW-MRI benefit a broader population of patients facing HNSCC.
Charting the Path for Future Innovations
The insights gained from current research on DW-MRI-guided therapy lay a strong foundation for exploring new frontiers in cancer treatment, opening doors to further enhancements in efficacy and safety. Combining DW-MRI with other emerging imaging modalities or molecular biomarkers could provide an even more comprehensive view of tumor biology, refining dose painting to unprecedented levels of accuracy. Additionally, integrating this approach with novel therapies such as immunotherapy or targeted agents holds potential to create synergistic effects, attacking cancer from multiple angles. Such multimodal strategies could amplify treatment success, addressing limitations that single-method approaches might encounter.
Prospective studies with larger cohorts and extended follow-up periods are also necessary to solidify the long-term benefits of DW-MRI-guided DP-IMRT, particularly regarding overall survival outcomes that remain inconclusive in shorter timeframes. Addressing logistical barriers, such as equipment availability and staff training, will be crucial to translating these innovations into everyday practice. As research progresses from 2025 onward, the focus should remain on scalability and accessibility, ensuring that future advancements build on current successes to redefine care standards. These steps forward promise to deepen the impact of precision oncology, offering renewed hope for better management of complex cancers like HNSCC.