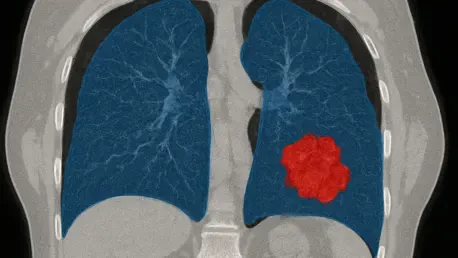
Imagine a scenario where the painstaking process of identifying lung cancer tumors in CT scans is transformed into a swift, precise, and automated task, dramatically improving treatment outcomes. A groundbreaking study recently published in BMC Cancer unveils D-S-Net, an advanced deep learning framework engineered to redefine the segmentation of Gross Tumor Volumes (GTVs) in lung cancer imaging. This critical step in radiotherapy planning has historically been hindered by the complexity of tumor shapes and the labor-intensive nature of manual delineation by radiologists. D-S-Net emerges as a potential game-changer, promising to address these persistent challenges with a novel dual-stage approach that could enhance accuracy and efficiency in oncology. By reducing human error and variability, this technology aims to pave the way for more effective treatment plans, ultimately offering hope for better patient care. The implications of such an innovation extend beyond mere technical advancement, hinting at a future where clinical workflows are streamlined and precision in cancer treatment becomes the norm.
Addressing the Complexities of Lung Cancer Imaging
Lung cancer presents formidable obstacles in medical imaging, primarily due to the vast diversity in tumor morphology and the often indistinct boundaries visible in CT scans. Tumors can vary significantly in size, shape, and texture, making it difficult to distinguish them from surrounding healthy tissue. Small or poorly defined tumors pose an even greater challenge, frequently leading to misinterpretation or oversight in automated systems. Historically, radiologists have had to manually outline these regions, a process that demands extensive time and expertise while remaining susceptible to inconsistencies across different practitioners. Such variability can directly impact the accuracy of radiotherapy planning, where precise tumor delineation is paramount. D-S-Net steps into this critical gap, aiming to automate segmentation with a level of precision that could standardize outcomes and minimize errors, thereby supporting more reliable treatment strategies in clinical settings.
Beyond the inherent difficulties of tumor characteristics, the high-resolution nature of modern CT scans adds another layer of complexity to the segmentation process. These detailed images, while invaluable for diagnosis, require sophisticated algorithms to process vast amounts of data without losing critical information. Traditional methods often struggle to balance detail with computational demands, resulting in either reduced accuracy or prolonged processing times. This challenge is particularly acute in busy hospital environments where efficiency is as crucial as precision. D-S-Net’s approach offers a potential solution by leveraging advanced deep learning techniques to handle high-resolution data more effectively. By focusing on targeted areas of interest, it seeks to overcome the limitations of older systems, providing a tool that could significantly reduce the burden on medical professionals while ensuring that no vital details are missed during the segmentation process.
Shortcomings of Existing Segmentation Technologies
Prior to the advent of D-S-Net, many deep learning models, such as SwinU-Net, demonstrated potential in tumor segmentation but often fell short when dealing with the intricacies of high-resolution CT images. A common issue was the need to downscale images to manage computational load, which inevitably led to a loss of fine details essential for accurate tumor boundary identification. Alternatively, models that attempted to process entire images at once frequently failed to prioritize specific tumor regions, resulting in suboptimal performance. These limitations underscored a pressing need for a more refined approach that could maintain image integrity while managing processing demands. The inefficiencies of these earlier systems often meant that radiologists still had to intervene manually, negating much of the intended benefit of automation in clinical practice.
Furthermore, the inability of existing models to adequately address class imbalance in medical imaging data compounded their shortcomings. In CT scans, tumor pixels are typically far outnumbered by background pixels, leading to biased models that overlook smaller or less prominent tumor areas. This imbalance can critically undermine the reliability of segmentation, especially for early-stage cancers where tumors may be subtle. The computational inefficiencies and lack of focus in prior technologies also meant longer processing times, which are impractical in real-world healthcare settings where rapid decision-making is often necessary. D-S-Net aims to rectify these issues with a design that not only enhances accuracy but also prioritizes speed and resource efficiency, potentially setting a new benchmark for automated segmentation tools in oncology imaging.
Exploring the Dual-Stage Innovation of D-S-Net
At the forefront of D-S-Net’s promise is its dual-stage architecture, a design that distinctly separates the processes of tumor detection and segmentation for optimal results. In the initial stage, a dedicated detection network scans high-resolution 512×512 pixel CT slices to identify candidate tumor regions, effectively narrowing down the data that needs detailed analysis. This targeted approach significantly reduces computational overhead without sacrificing the integrity of the image data. Following this, the second stage employs a customized U-Net architecture enhanced with a spatial attention mechanism to focus on refining tumor boundaries with remarkable precision. By mimicking the diagnostic workflow of expert clinicians who first identify suspicious areas before detailed examination, D-S-Net offers a methodical and efficient solution to the challenges of lung cancer imaging.
Additionally, D-S-Net incorporates a hybrid loss function that combines binary cross-entropy and Dice loss to tackle the pervasive issue of class imbalance in medical images. This innovative feature ensures that the model remains balanced in its optimization, giving due attention to underrepresented tumor pixels amidst a sea of background data. Such a mechanism is crucial for achieving high pixel-wise accuracy and better overlap with actual tumor regions. The synergy of these components—detection, attention, and balanced loss—positions D-S-Net as a forward-thinking tool that not only improves segmentation outcomes but also aligns with the practical needs of clinical environments. This thoughtful integration of features suggests a significant leap forward, potentially reducing the time and variability associated with manual delineation while enhancing the overall reliability of tumor mapping for treatment planning.
Measuring Success Through Performance Data
The performance of D-S-Net has been rigorously tested across two distinct datasets, yielding results that highlight its transformative potential in tumor segmentation. On the primary lung cancer GTV dataset, the model recorded a Dice coefficient of 78.52%, surpassing the SwinU-Net model by a notable margin of over 5%. This metric, which evaluates the overlap between predicted and actual tumor regions, reflects a substantial improvement in accuracy. Even more striking was the performance on an independent dataset, where D-S-Net achieved a Dice coefficient of 86.56%, outpacing competitors by more than 13%. These figures, supported by comprehensive validation and ablation studies, confirm the critical role of each component in the model’s design, from the detection stage to the spatial attention mechanism, in driving superior outcomes.
Moreover, the consistency of D-S-Net’s performance across varied datasets speaks to its robustness and adaptability, key factors for real-world application. The significant improvements in Dice coefficients indicate not just a technical achievement but a practical advancement that could influence patient care by ensuring more precise radiotherapy targeting. Validation through ablation studies further revealed how the hybrid loss function contributes to balanced optimization, addressing common pitfalls in medical imaging where small tumors might otherwise be missed. These results collectively suggest that D-S-Net is not merely an incremental upgrade over existing models but a potential standard-setter in the field. The data underscores the possibility of reducing diagnostic errors and enhancing treatment efficacy, marking a pivotal moment in the evolution of automated segmentation technologies for oncology.
Transforming Clinical Practice with Real Benefits
The implications of D-S-Net extend far beyond impressive metrics, offering substantial benefits for clinical practice in lung cancer treatment. Accurate segmentation of GTVs is a cornerstone of effective radiotherapy planning, directly affecting the ability to deliver precise radiation doses while minimizing harm to surrounding healthy tissues. By automating this process with high precision, D-S-Net has the potential to alleviate the workload of radiologists, who often spend considerable time manually outlining tumors. This reduction in manual effort could lead to faster diagnosis and treatment initiation, a critical factor in improving patient outcomes, especially in aggressive cancers where timing is essential. Additionally, the consistency offered by automated segmentation may help standardize practices across different medical facilities.
Equally important is D-S-Net’s capacity to support real-time decision-making, particularly in adaptive radiotherapy where treatment plans must be adjusted on the fly based on changing tumor characteristics. The model’s ability to deliver quick and reliable segmentation results could enable clinicians to make informed adjustments during treatment sessions, enhancing therapeutic effectiveness. Furthermore, by minimizing inter-observer variability—a common issue when multiple radiologists interpret the same scans—D-S-Net could foster greater uniformity in tumor delineation, ensuring that patients receive consistent care regardless of who oversees their imaging. These clinical advantages highlight the model’s potential to not only improve individual patient care but also elevate the overall quality of oncology services in healthcare systems facing increasing demands.
Balancing Precision with Computational Practicality
A standout attribute of D-S-Net is its emphasis on computational efficiency, a crucial consideration for integration into hospital workflows. The dual-stage approach ensures that resources are focused on relevant tumor regions rather than processing entire high-resolution images unnecessarily. This targeted strategy reduces the computational burden, making the model faster and more scalable compared to predecessors that struggled with processing times. In busy clinical settings where equipment and time are often limited, such efficiency translates into practical benefits, allowing for quicker turnaround in imaging analysis without compromising on the accuracy needed for effective treatment planning. D-S-Net’s design aligns with the real-world constraints of healthcare environments, positioning it as a viable tool for widespread adoption.
In addition to speed, the model’s compatibility with existing systems further enhances its practicality. Many hospitals operate under strict technological and budgetary constraints, making the adoption of new tools challenging if they require significant infrastructure changes. D-S-Net’s efficient use of computational resources means it can likely be integrated into current setups with minimal disruption, facilitating a smoother transition to automated segmentation. This balance of precision and practicality addresses a critical barrier to the implementation of AI in medicine, where innovations must not only perform well in controlled studies but also prove feasible in day-to-day operations. The focus on resource optimization suggests that D-S-Net could play a key role in making advanced imaging technologies more accessible, particularly in under-resourced facilities striving to improve cancer care.
Envisioning a Future for AI in Oncology
The development of D-S-Net mirrors a broader movement in oncology toward harnessing artificial intelligence to solve intricate diagnostic and therapeutic challenges. Its success in lung cancer segmentation hints at possibilities for broader applications, potentially aiding in the precise mapping of tumors in other cancer types where imaging complexities are similar. As AI tools become increasingly sophisticated, their integration into routine medical practice appears inevitable, promising to enhance the accuracy and speed of critical processes like tumor delineation. D-S-Net exemplifies how domain-specific innovations, such as spatial attention mechanisms and hybrid loss functions, can address nuanced problems in medical imaging, setting a precedent for future advancements that could reshape cancer care on a global scale.
Looking ahead, the trajectory of D-S-Net suggests a need for continued exploration into multi-modal imaging data, such as combining CT with PET or MRI scans, to further refine tumor characterization. Expanding the model to handle 3D volumes could also provide a more comprehensive spatial understanding, enhancing its utility in complex cases. Prospective clinical trials will be essential to validate its impact on patient outcomes and ensure seamless integration into diverse healthcare settings. As the field of oncology continues to embrace AI, D-S-Net stands as a beacon of what targeted innovation can achieve, inspiring further research and development to bridge the gap between technological potential and tangible improvements in patient care. This forward-looking perspective underscores the transformative role that such tools could play in the ongoing fight against cancer.