Navigating the complexities of brain cancer treatment often involves a perilous journey through diagnostic uncertainty, where distinguishing between tumor regrowth and treatment-related side effects can be a formidable challenge for clinicians. In a significant move to address this critical need, Telix Pharmaceuticals has officially submitted a marketing authorization application to European regulators for its investigational imaging agent, TLX101-Px. This radiopharmaceutical is specifically designed to enhance the visualization of glioma, a prevalent and aggressive form of brain cancer. The submission marks a pivotal moment for the company and holds the promise of introducing a more precise diagnostic tool that could fundamentally alter how neuro-oncologists monitor disease and guide patient care. By potentially offering a clearer view of tumor activity at a biological level, this technology aims to empower medical professionals with the confidence needed to make timely and effective treatment decisions, potentially improving outcomes for patients battling this devastating disease.
Innovations in Neuro-Oncology Imaging
Addressing Diagnostic Ambiguity
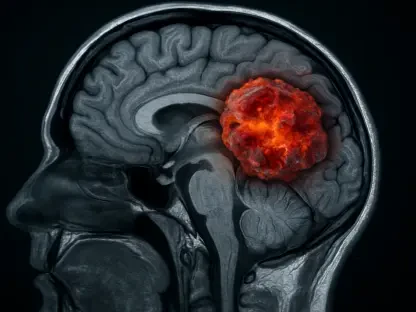
One of the most persistent challenges in managing glioma, which originates in the supportive glial cells of the brain, is the difficulty in accurately assessing disease status after initial treatment. Standard imaging modalities like magnetic resonance imaging (MRI) and positron emission tomography (PET) scans are staples in neuro-oncology, yet they possess inherent limitations. A primary issue is distinguishing true tumor progression from pseudo-progression—a phenomenon where brain tissue appears to be growing on a scan due to treatment effects like inflammation and radiation necrosis, rather than actual cancer cell proliferation. This ambiguity can lead to critical delays in appropriate care or, conversely, to premature and unnecessary changes in therapy, including invasive procedures. The ability to differentiate between these two states is paramount for effective patient management, as a misinterpretation can significantly impact treatment pathways and overall prognosis. TLX101-Px has been developed to directly confront this diagnostic gray area, offering a more refined method for evaluating the underlying biology of the suspected lesion.
The Mechanism of Action
At the core of TLX101-Px’s innovative approach is its chemical composition, O-(2-[18F]fluoroethyl)-L-tyrosine (18F-FET), a PET scan tracer designed with a high degree of biological specificity. Unlike conventional imaging techniques that primarily visualize structural changes, this agent functions by targeting key molecular pathways associated with cancer growth. It is engineered to bind to the L-type amino acid transporter types 1 and 2 (LAT1 and LAT2), proteins that are frequently overexpressed on the surface of glioma cells to fuel their rapid proliferation. By accumulating in areas with high LAT1 and LAT2 activity, the radiopharmaceutical effectively “lights up” active tumor tissue during a PET scan. This allows clinicians to see a metabolic and biological map of the cancer, rather than just its anatomical outline. As affirmed by Dr. Philipp Lohmann, a respected neuroimaging expert from Germany, this advanced technology is poised to provide “greater biological insight” precisely where it is most needed, enabling a more accurate and confident assessment of tumor activity.
Strategic Implications and Future Outlook
The Theranostic Approach
The development and regulatory submission of TLX101-Px are integral components of Telix’s broader corporate vision, which centers on a “theranostic” paradigm. This strategy involves the parallel development of paired diagnostic and therapeutic agents that target the same molecular marker, creating a highly personalized and targeted approach to cancer treatment. In this case, the imaging agent, TLX101-Px, serves as the diagnostic guide for its therapeutic counterpart, TLX101-Tx (iodofalan 131I). The therapeutic agent is a small molecule that also binds to the LAT1 protein but is armed with a potent radioactive isotope, iodine-131. The overarching goal is to first use the imaging agent to identify patients whose tumors exhibit the LAT1 target, confirming they are suitable candidates for the therapy. Subsequently, the therapeutic agent can be administered to deliver a precise and lethal dose of radiation directly to the cancer cells while minimizing damage to surrounding healthy brain tissue. This synergistic relationship is fundamental to the company’s strategy in neuro-oncology.
Global Regulatory and Clinical Trajectory
The European filing for TLX101-Px represents a crucial step in establishing a global footprint for Telix’s glioma imaging program, which directly underpins its therapeutic development efforts. Kevin Richardson, CEO of the company’s diagnostics subsidiary, highlighted that this submission is key to building widespread access and utilization, which in turn supports the patient selection pipeline for the corresponding therapy. Furthermore, the company has confirmed its intention to submit a similar application to the U.S. Food and Drug Administration (FDA) in the near future, noting that preparations for the FDA submission streamlined and expedited the European filing process. Concurrently, the therapeutic agent, TLX101-Tx, is advancing through late-stage clinical evaluation. It is currently the subject of the IPAX BrIGHT Phase 3 trial (NCT07100730), which is actively recruiting patients in Australia with recurrent glioblastoma. This pivotal study is designed to assess the safety and efficacy of the therapy when added to the existing standard-of-care treatments for this aggressive cancer.
Pioneering a New Standard of Care
The submission of the marketing application to European authorities marked a culmination of extensive research and development aimed at addressing a clear, unmet need in neuro-oncology. The potential approval of TLX101-Px promised to equip clinicians with a sophisticated tool capable of providing deeper biological insights into glioma activity. This advancement was positioned not only as an improvement in diagnostic accuracy but also as a foundational element of a larger, integrated theranostic strategy. The successful deployment of this imaging agent was seen as the first step toward a future where treatment could be more precisely tailored to individual patient biology. The ongoing clinical trials for its therapeutic partner, TLX101-Tx, further underscored a commitment to transforming the entire continuum of glioma care, from initial diagnosis and monitoring to targeted, personalized therapy.